Nature Chemistry


For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/asia.201590003/abstract
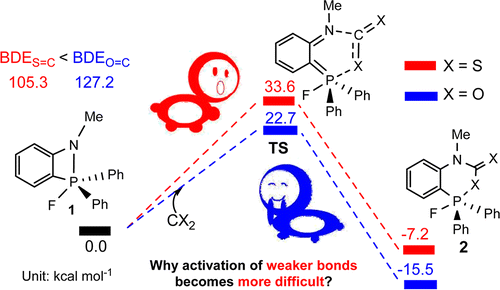
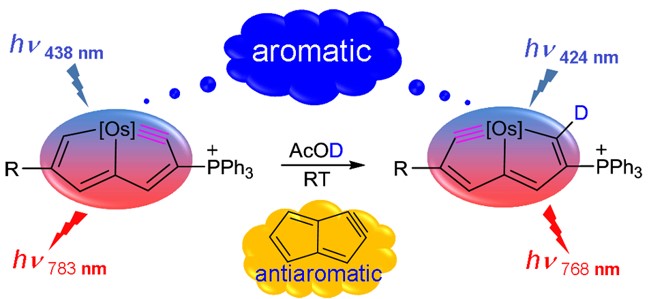
The sequestration of carbon disulfide (CS2), a common pollutant in environmental systems, is of great importance due to its physical harm to human beings. Compared with CO2 capture, that of CS2 is much less developed. The use of P/N-based frustrated Lewis pairs (FLPs) has been proven, both experimentally and theoretically, to be an alternative strategy to efficiently sequestrate CO2. Therefore, we pose the question of whether the analogue CS2 could also be sequestrated by the same FLPs, given that the C═S bond in CS2 is weaker than the C═O bond in CO2.
Dr. Zhu introduced the group projects to Prof. Pierre H. Dixneuf in his office on Dec 1st, 2014.
Congratulations!
Ms. Xuerui Wang is awarded the National Graduate Studentship. Congratulations!
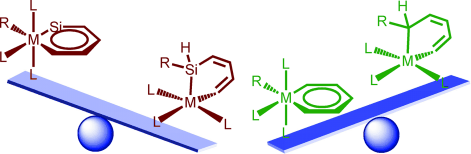
Density functional theory (DFT) calculations were carried out to investigate the 1,2-migration in metallasilabenzenes. The results suggested that the chloride migration of metallabenzenes is unfavorable due to the loss of aromaticity in the nonaromatic analogues. In sharp contrast, such a migration in metallasilabenzenes is favorable due to the reluctance of silicon to participate in π bonding. The migration of hydride and methyl group from the metal center to the silicon atom in metallasilabenzenes is computed to be also feasible.
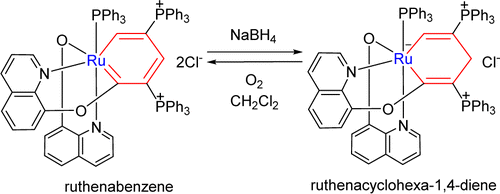
Treatment of ruthenabenzene [(C9H6NO)Ru{CC(PPh3)CHC(PPh3)CH}(C9H6NO)(PPh3)]Cl2 (1) with NaBH4 produces the first ruthenacyclohexa-1,4-diene [(C9H6NO)Ru{CC(PPh3)CH2C(PPh3)CH}(C9H6NO)(PPh3)]Cl (2), which was fully characterized. Under an oxygen atmosphere, complex 2 can easily convert to complex 1. DFT calculations were carried out to rationalize the high regioselectivity in the reaction of the ruthenabenene 1 with NaBH4 and the interconversion between 1 and 2.
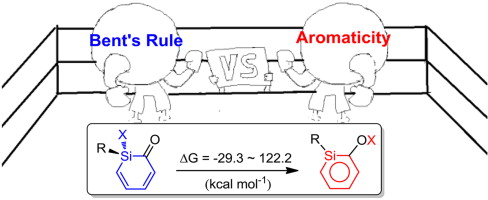
Density functional theory (DFT) calculations were performed to examine the substituent effects on the interconversion of silabenzenes and their monocyclic non-aromatic isomers. A previous study suggested that aromaticity is the driving force for this process. Interestingly, our systematic calculations reveal that the contribution from aromaticity can be evaluated quantitatively (ca. 30 kcal mol-1). Thus it is the interplay of aromaticity and Bent's rule that determine their relative stabilities.
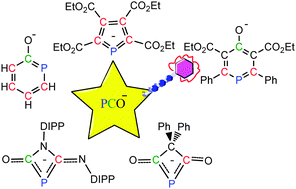
Density functional theory (DFT) calculations were carried out to investigate the [2+2], [3+2] and [4+2] cycloadditions of the phosphaethynolate anion (PCO−). The results reveal that the electronic properties of different unsaturated compounds play a crucial role in reactivity and regioselectivity.
http://pubs.rsc.org/en/Content/ArticleLanding/2014/CC/C4CC04610B#!divAbstract
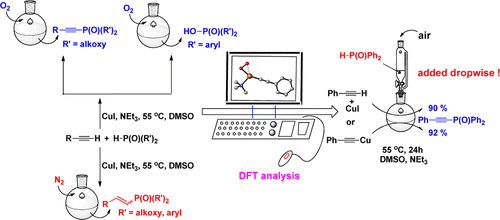
The reaction mechanism of copper-catalyzed phosphorylation of terminal alkynes under different conditions has been investigated experimentally and theoretically. The important role of dioxygen has been elucidated, including the formation of η1-superoxocopper(II), η2-superoxocopper(III), μ-η2:η2-peroxodicopper(II), and bis(μ-oxo)dicopper(III) complexes.
Copyright © 2025,
Theme Originally Created by Devsaran
