Nature Chemistry


The degree of p-electron (de)localization and aromaticity of a series of polybenzenoid hydrocarbons (PBHs) has been analyzed through the π-contribution to the electron localization function (ELFπ), calculated at the B3LYP/6-311G(d,p) hybrid density functional theory level. The extent of p-electron delocalization in the various hexagons of a PBH was determined through analysis of the bifurcation values of the ELFp basins (BV(ELFp)), the spans in the bifurcation values in each hexagon (ΔBV(ELFπ)), and the ring-closure bifurcation values of the ELFπ (RCBV(ELFπ)).
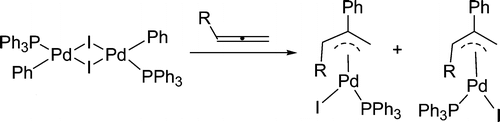
Treatment of [PdI(Ph)(PPh3)]2 with allenes CH2═C═CHR (R = CMe3, CO2Et, P(O)(OEt)2, and SO2Ph) in dichloromethane at room temperature produces a mixture of cis and trans isomers of the π-allyl palladium complexes PdI(η3-CH2C(Ph)CHR)(PPh3) in which the R group is anti to the Ph group. The disubstituted allenes MeCH═C═CHR (R = P(O)(OEt)2 and SO2Ph) similarly react with [PdI(Ph)(PPh3)]2 to give the π-allyl palladium complexes PdI(η3-MeCHC(Ph)CHR)(PPh3) in which the R group is anti and the Me group is syn to the Ph group.
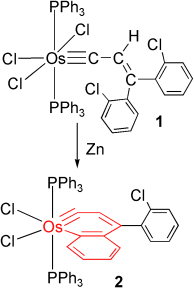
Cl prevents insertion: The first metallanaphthalyne 2 has been obtained by Zn reduction of Os carbyne complex 1. The key to its isolation was the use of o-chlorophenyl instead of phenyl substituents to avoid formation of a putative hydrido metallanaphthalyne intermediate (supported by DFT calculations), which undergoes migratory insertion of the carbyne into the OsH bond and rearrangement to give an indenyl complex as the final product.
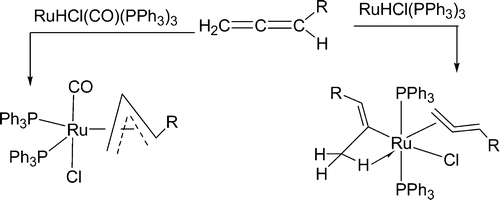
Treatment of RuHCl(CO)(PPh3)(3) with CH2=C=CHCO2Me gives the allyl complex Ru(77 3 -CH2CHCHCO2Me)CI(CO)(PPh3)(2). The analogous allyl complexes Os(eta(3)-CH2CHCHR)Cl(CO)(PPh3)(2) (R = Ph, CH2Ph) are also produced from the reactions of OsHCI(CO)(PPh3)(3) with CH2=C=CHR. In contrast, MHCl(PPh3)(3) (M = Ru, Os) react with CH2=C=CHR to give the vinyl complexes MCl((C(CH3)=CHR)(CH2 C=CHR)(PPh3)(2) (M = Ru, R = CMe3, M = Os, R = CMe3, Ph, CO2Et).
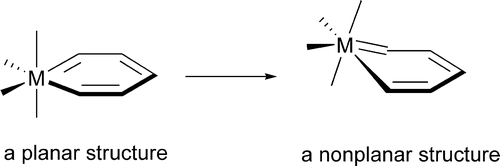
The nonplanarity found in metallabenzene complexes has been investigated theoretically via density functional theory (DFT) calculations. A metallabenzene has four occupied π molecular orbitals (8 π electrons) instead of three that benzene has. Our electronic structure analyses show that the extra occupied π molecular orbital, which is the highest occupied molecular orbital (HOMO) in many metallabenzenes, has antibonding interactions between the metal center and the metal-bonded ring-carbon atoms, providing the electronic driving force toward nonplanarity.
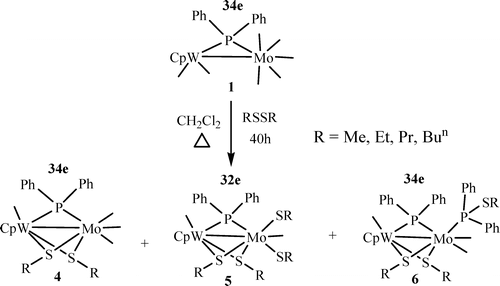
Reactions of CpW(CO)(2)(mu-PPh2)Mo(CO)(5) (1) with alkyl disulfides RSSR (R = Me, Et, Pr, Bu-n) in refluxing dichloromethane yielded the series of new mixed-metal and mixed-ligand bridged compounds CpW(CO)(mu-SR)(2)(mu-PPh2)Mo(CO)(3) (R = Me (4a), Et (4b), Pr (4c), Bun (4d)), CpW(CO)(mu-SR)2(mu-PPh2)Mo(CO)(mu-SR)(2) (R = Me (5a), Et (5b), Pr (5c), Bu-n (5d)), and CpW(CO)(mu-SR)(2)(mu-PPh2) Mo(CO)(2)(PPh2SR) (R = Me (6a), Et (6b), Pr (6c), Bu-n (6d)). All except 6c were characterized by single-crystal X-ray diffraction analysis.
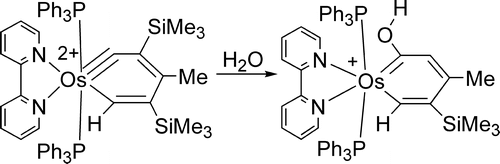
Treatment of the osmabenzyne Os(equivalent to CC(SiMe3)=C(Me)C(SiMe3)=CH)Cl-2(PPh3)(2) (1) with 2,2'-bipyridine (bipy) and thallium triflate (TlOTf) produces the thermally stable dicationic osmabenzyne [Os( equivalent to CC(SiMe3)=C(Me)C(SiMe3)=CH)(bipy)(PPh3)(2)](OTf)(2) (2). The dicationic osmabenzyne 2 reacts with ROH (R = H, Me) to give osmabenzene complexes [Os(=C(OR)CH=C(Me)C(SiMe3)=CH)(bipy)(PPh3)(2)]OTf, in which the metallabenzene ring deviates significantly from planarity.
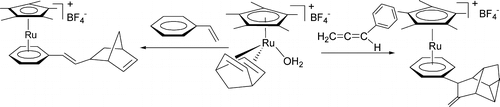
Formal [2+2+2] addition reactions of the NBD ligand in [Cp*Ru(H2O)(NBD)]BF4 (NBD = norbornadiene) with H-2, Ph3SiH, ArCH=C=CH2, and RC=-CPh were observed. In contrast, olefins such as styrene and NBD do not undergo similar [2+2+2] addition reactions with [Cp*Ru(H2O)(NBD)]BF4. [Cp*Ru(H2O)(NBD)]BF4 reacts with H-2 in benzene to give [Cp*Ru(eta(6)-C6H6)]BF4 and nortricyclene. Similarly, [Cp*Ru(H2O)(NBD)]BF4 reacts with Ph3SiH to give [Cp*Ru(eta(6)-C6H5SiPh2OH)]BF4 and nortricyclene.
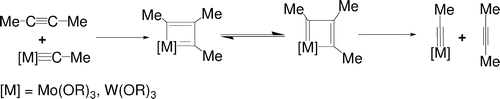
In this paper, the mechanism of alkyne metathesis catalyzed by W/Mo alkylidyne complexes has been theoretically investigated with the aid of density functional theory calculations. Calculations on various model alkylidyne complexes M( CMe)(OR)(3) (M = W, Mo; R = Me, CH2F), W( CMe)(NMe2)(3), and W( CMe)(Cl)(3) allow us to examine the factors that influence the reaction barriers. In the reaction mechanism, metallacyclobutadienes are initially formed from a ring-closing step between alkynes and alkylidyne complexes. A ring-opening step then gives the metathesis products.
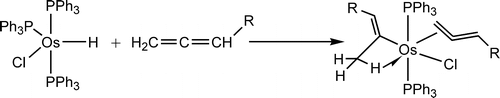
Treatment of OsHCl(PPh3)(3) with allenes CH2=C=CHR at room temperature in benzene produced the vinyl complexes OsCl(C(CH3)=CHR)(CH2=C=CHR)-(PPh3)(2), instead of eta(3)-allyl complexes as normally observed. DFT calculations show that the formation of the vinyl complex is favored kinetically.
Copyright © 2025,
Theme Originally Created by Devsaran
