Nature Chemistry
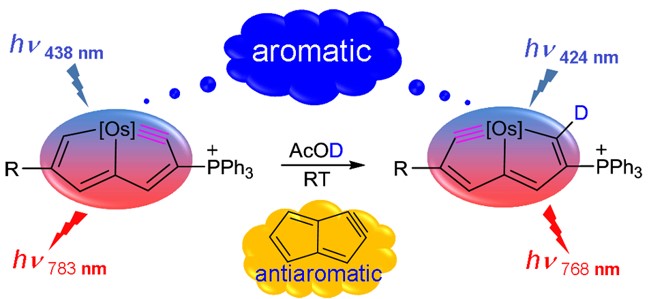


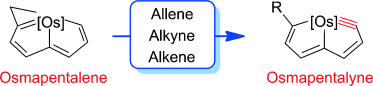
The synthesis of small cyclic metal carbynes is challenging due to the large angle strain associated with the highly distorted nonlinear triple bonds. Herein, we report a general route for the synthesis of five-membered cyclic metal carbyne complexes, osmapentalynes, by the reactions of an osmapentalene derivative with allene, alkyne, and alkene. Experimental observations and theoretical calculations document the aromaticity in the fused five-membered rings of osmapentalynes.
For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/anie.201502412/abstract.

For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/anie.201503578/abstract
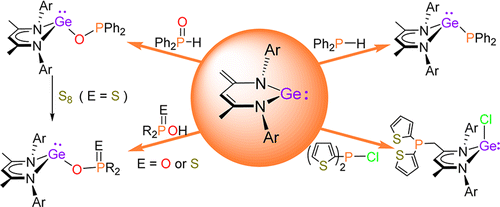
A series of phosphorus-substituted germanium(II) complexes, L1GeR [L1 = CH{(CMe)(2,6-iPr2C6H3N)}2; 2, R = PPh2; 4, R = OPPh2; 5a, R = OP(O)Ph2; 5b, R = OP(O) (OnBu)2; 6a, R = OP(S)Ph2; 6b, R = OP(S)(OEt)2], were synthesized through the direct activation of various organic phosphorus compounds by N-heterocyclic ylide-like germylene 1.
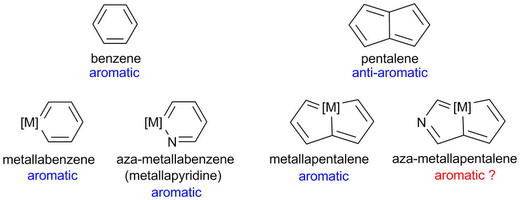
The concept of aromaticity has long played an important role in chemistry and continues to fascinate both experimentalists and theoreticians. Among the archetypal aromatic compounds, heteroaromatics are particularly attractive. Recently, substitution of a transition-metal fragment for a carbon atom in the anti-aromatic hydrocarbon pentalene has led to the new heteroaromatic osmapentalenes. However, construction of the aza-homolog of osmapentalenes cannot be accomplished by a similar synthetic manipulation.
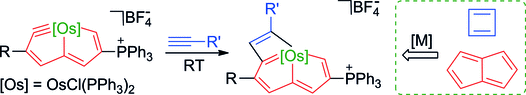
Antiaromatic species are substantially less thermodynamically stable than aromatic moieties. Herein, we report the stabilization of two classical antiaromatic frameworks, cyclobutadiene and pentalene, by introducing one metal fragment through the first [2+2] cycloaddition reaction of a late-transition-metal carbyne with alkynes. Experimental observations and theoretical calculations reveal that the metal fragment decreases the antiaromaticity in cyclobutadiene and pentalene simultaneously, leading to air- and moisture-stable products.
For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/anie.201501349/abstract
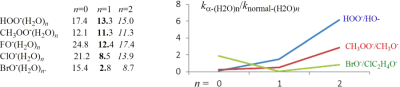
To probe the kinetic performance of microsolvated α-nucleophile, the G2(+)M calculations were carried out for the gas-phase SN2 reactions of monohydrated and dihydrated α-oxy-nucleophiles XO−(H2O)n = 1,2 (X = HO, CH3O, F, Cl, Br), and α-sulfur-nucleophile, HSS−(H2O)n = 1,2, toward CH3Cl. We compared the reactivities of hydrated α-nucleophiles to those of hydrated normal nucleophiles.
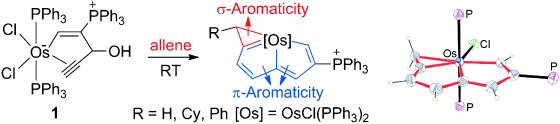
In general, aromaticity can be clarified as π- and σ-aromaticity according to the type of electrons with major contributions. The traditional π-aromaticity generally describes the π-conjugation in fully unsaturated rings whereas σ-aromaticity may stabilize fully saturated rings with delocalization caused by σ-electron conjugation. Reported herein is an example of σ-aromaticity in an unsaturated three-membered ring (3 MR), which is supported by experimental observations and theoretical calculations.
For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/anie.201411220/abstract
Please note that it is the calculations that discover the σ-Aromaticity in such a uniqu system.
Copyright © 2025,
Theme Originally Created by Devsaran
