Nature Chemistry


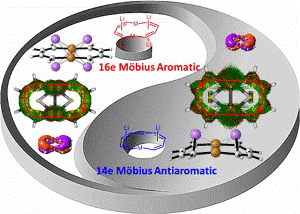
Aromaticity, one of the most fundamental concepts in chemistry, can be classified as Hückel- and Möbius-type according to the electron count and topology. In comparison with numerous Hückel aromatics containing 4n+2 π-electrons, Möbius aromatics with 4n π-electrons, especially the Craig-type species are particularly limited.
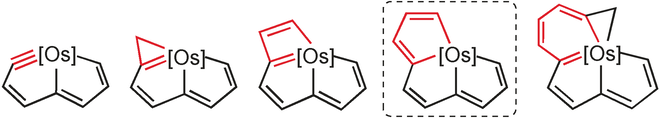
Polycyclic complexes containing a bridgehead transition metal are interesting species because the transition metal is shared by all the rings simultaneously. In this study, we present a novel osmium–bridgehead system with three fused five-membered rings. This novel framework can be viewed as a 10-atom carbon chain coordinating to the osmium center. In sharp contrast to the nonplanar organic analogue, this unique metallacycle exhibits good planarity, which was unambiguously verified by means of X-ray diffraction.
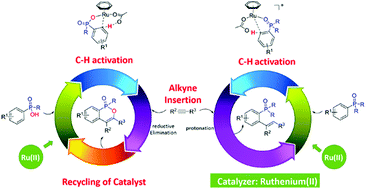
Using density functional theory (DFT) calculations, the present study explores the mechanisms of two ruthenium(II)-catalyzed phosphoryl-directed ortho-selective C–H bond activation reactions. Depending on the nature of the phosphoryl groups, namely R2P(O) versus RP(O)OH, two different products could be selectively synthesized. For R2P(O), the overall catalytic cycle includes three basic steps: C–H bond activation, alkyne insertion, and protonation. The oxidation state of the Ru center does not change during this catalytic process.

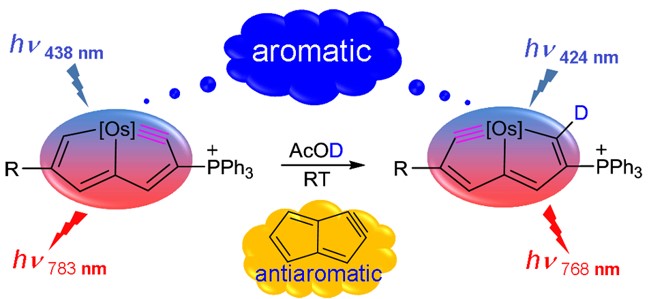
Treatment of osmapentalyne [Os{≡C-C(COOMe)=CH-C=CH-C(PPh3)=CH-}Cl(PPh3)2]+BF4- with arylamines in the presence of Cs2CO3 produced osmium-bridged polycyclic aromatic complexes. In this reaction, metal carbyne of osmapentalyne was first attacked by nucleophiles, followed by a C-H oxidative addition. The UV-Vis spectra of these osmium-bridged polycyclic aromatic complexes were measured. The result shows that these osmium-bridged polycyclic aromatic complexes have broad absorption in the UV-Vis region up to 650 nm.
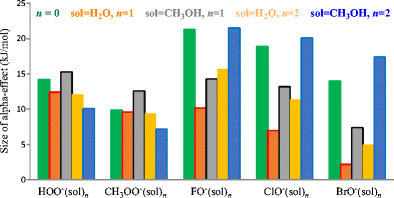
The modified G4(MP2) method was applied to explore microsolvation effects on the reactivity of four solvated normal oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = CH3, C2H5, FC2H4, ClC2H4), and five α-oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = HO, CH3O, F, Cl, Br), in gas-phase SN2 reactions towards the substrate CH3Cl.

Metalla-aromatics are attractive species because they exhibit the properties of both organometallics and aromatics. Reported metal-bridged polycyclic aromatic complexes, as well as Möbius aromatic species, are still rare. Herein, we present the construction of two new metal-bridged polycyclic aromatic frameworks, α-metallapentalenofurans and lactone-fused metallapentalynes, by the reactions of osmapentalyne with terminal aryl alkynes in the presence of H2O or HBF4/H2O, respectively.
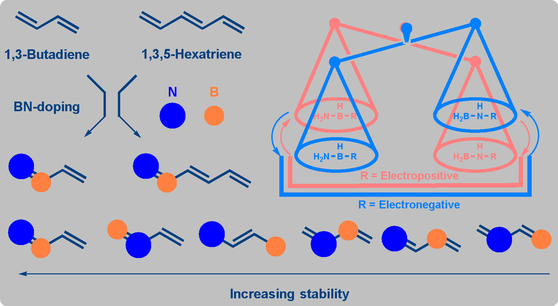
The BN-doped organic analogues are interesting as aliphatic amineboranes for hydrogen storage, precursors for aromatic borazines and adsorbent cage azaboranes. However, BN-doped aliphatic polyenes remained undeveloped. Herein, we perform theoretical calculations on two mono BN-doped aliphatic lower polyenes, 1,3-butadiene and 1,3,5-hexatriene. A general rule is proposed, i.e., isomers with terminal nitrogen and directly BN-connected, N−B(R), in particular, are of significant thermodynamic stability as compared with their inverse analogues (where boron is at the terminal position).
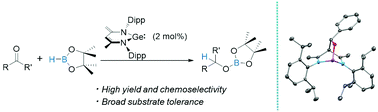
N-heterocyclic ylide-like germylene effectively promotes the hydroboration of aldehydes and ketones under mild conditions with broad substrate tolerance, operational simplicity of procedure and excellent yields. A key intermediate in this catalytic system featuring a bicyclo[2,2,2]octane-like core has been successfully isolated and characterized, suggesting a new type of mechanism that involves the activation mode that mimics that of transition metal catalysts.
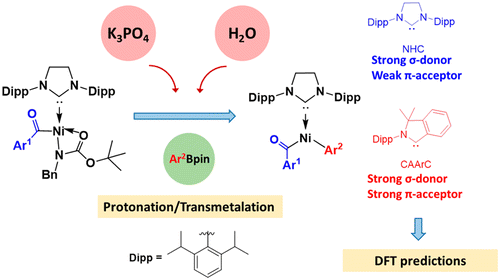
In textbooks, the low reactivity of amides is attributed to the strong resonance stability. However, Garg and co-workers recently reported the Ni-catalyzed activation of robust amide C–N bonds, leading to conversions of amides into esters, ketones, and other amides with high selectivity. Among them, the Ni-catalyzed Suzuki-Miyaura coupling (SMC) of N-benzyl-N-tert-butoxycarbonyl (N-Bn-N-Boc) amides with pinacolatoboronate (PhBpin) was performed in the presence of K3PO4 and water. Water significantly enhanced the reaction.
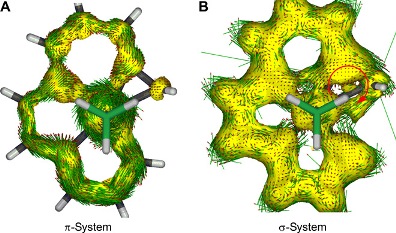
The coordinating atoms in polydentate chelates are primarily heteroatoms. We present the first examples of pentadentate chelates with all binding atoms of the chelating agent being carbon atoms, denoted as CCCCC chelates. Having up to five metal-carbon bonds in the equatorial plane has not been previously observed in transition metal chemistry. Density functional theory calculations showed that the planar metallacycle has extended Craig-Möbius aromaticity arising from 12-center–12-electron dπ-pπ π-conjugation.
Copyright © 2025,
Theme Originally Created by Devsaran
