Nature Chemistry



Fluorine is the most electronegative element in the periodic table. Thus, activation of the carbon–fluorine (C−F) bond, the strongest single bond to carbon, has attracted considerable interest from both experimentalists and theoreticians. In comparison with numerous approaches to activate C−F bonds, the aromaticity‐promoted method is less developed. Herein, we demonstrate that the C−F bond activation could be achieved by a facile tautomerization, benefitting from aromaticity, which can stabilize both the transition states and products.
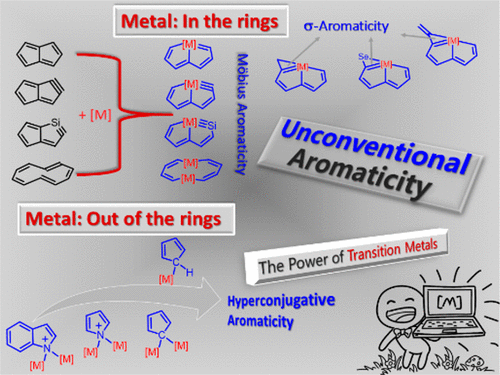
Aromaticity, one of the most fundamental concepts in chemistry, has attracted considerable attention from both theoreticians and experimentalists. Much effort on aromaticity in organometallics has been devoted to metallabenzene and derivatives. In comparison, aromaticity in other organometallics is less developed. This Account describes how our group has performed quantum chemical calculations to examine aromaticity in recently synthesized novel organometallic complexes.
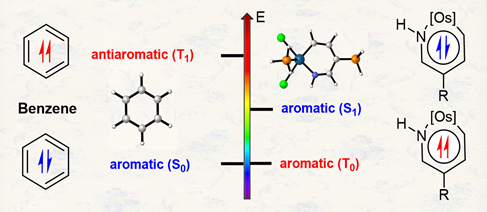
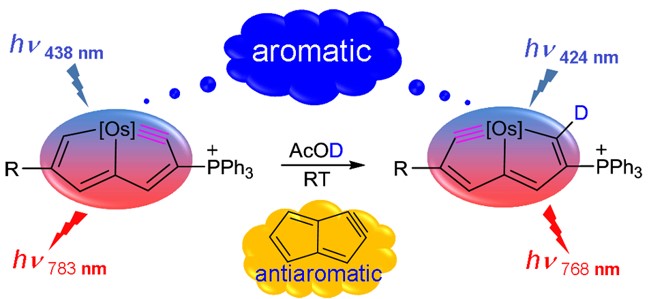
According to Hückel’s and Baird’s rules, cyclic conjugated species are aromatic either in the ground state or in the excited state only. Thus, species with aromaticity in both states (denoted as adaptive aromaticity) are particularly rare. Here we carry out density functional theory calculations on a series of osmapyridine and osmapyridinium complexes (96 species) and find that two of them display adaptive aromaticity, which was verified by various aromaticity indices including HOMA, ELFπ, MCI, ACIDπ plots and the heat of hydrogenation.
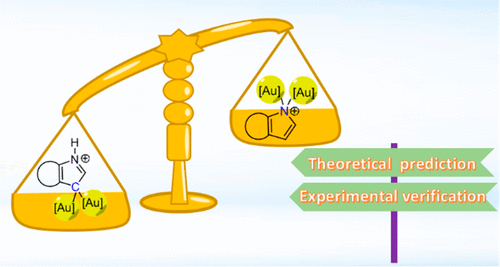
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. Although it has been extensively investigated in various reactions, the regioselectivity of hyperconjugative aromaticity on either main group systems or transition metal ones remains elusive due to the challenge of synthesizing the target products. Here we report a joint theoretical and experimental study on this issue.
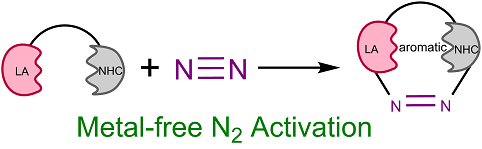
Molecular nitrogen (N2) is abundant in the atmosphere and nitrogen, found in many biomolecules, is an essential element of life. The Haber–Bosch process, developed over 100 years ago, requires relatively harsh conditions to activate N2 on the iron surface and generate ammonia for use as fertilizer or to produce other chemicals, leading to consumption of more than 2% of the world’s annual energy supply. Thus, developing approaches for N2 activation under mild conditions is particularly important and urgent.

Hyperconjugation and aromaticity are two of the most important concepts in chemistry. Mulliken and co‐workers combined both terms to explain the stability of cyclopentadiene. Here, we carried out DFT calculations on a series of mono‐ and disubstituted cyclopentadiene derivatives to investigate their hyperconjugative aromaticity. Our results revealed that one electropositive substituent can induce aromaticity, whereas one electronegative substituent prompts nonaromaticity rather than antiaromaticity.

Aromaticity is one of the most fundamental and fascinating chemical topics, attracting both experimental and theoretical chemists owing to its many manifestations. Both σ‐ and π‐aromaticity can be classified depending on the character of the cyclic electron delocalization. In general, σ‐aromaticity stabilizes fully saturated rings with σ‐electron delocalization whereas the traditional π‐aromaticity describes the π‐conjugation in fully unsaturated rings.

Rational design of a molecular catalyst for a hydrogen evolution reaction (HER) with both high rates and low overpotential remains a challenge. Here we report a series of Ni‐based catalysts incorporating non‐innocent ligands and aimed at reduction of the overpotential by reserving electrons in the ligand, which could decouple the correlation between metal center reduction and metal hydride formation.
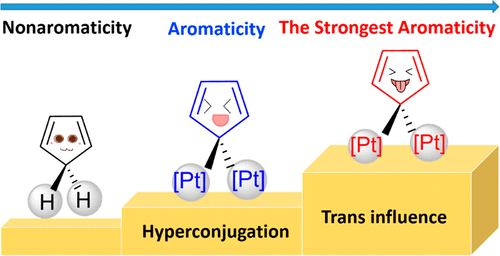
Hyperconjugation, an interaction of electrons in a σ orbital or lone pair with an adjacent π or even σ antibonding orbital, can have a strong effect on aromaticity. However, most work on hyperconjugative aromaticity has been limited to main-group substituents. Here, we report a thorough density functional theory study to evaluate the aromaticity in various cyclopentadienes that contain both main-group and transition-metal substituents.

Besides its mathematical importance, the Möbius topology (twisted, single-sided strip) is intriguing at the molecular level, as it features structural elegance and distinct properties; however, it carries synthetic challenges. Although some Möbius-type molecules have been isolated by synthetic chemists accompanied by extensive computational studies, the design, preparation, and characterization of stable Möbius-conjugated molecules remain a nontrivial task to date, let alone that of molecular Möbius strips assembling into more complex topologies.
Copyright © 2025,
Theme Originally Created by Devsaran
