Nature Chemistry
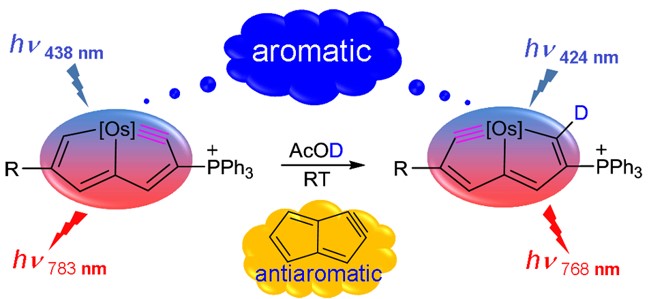


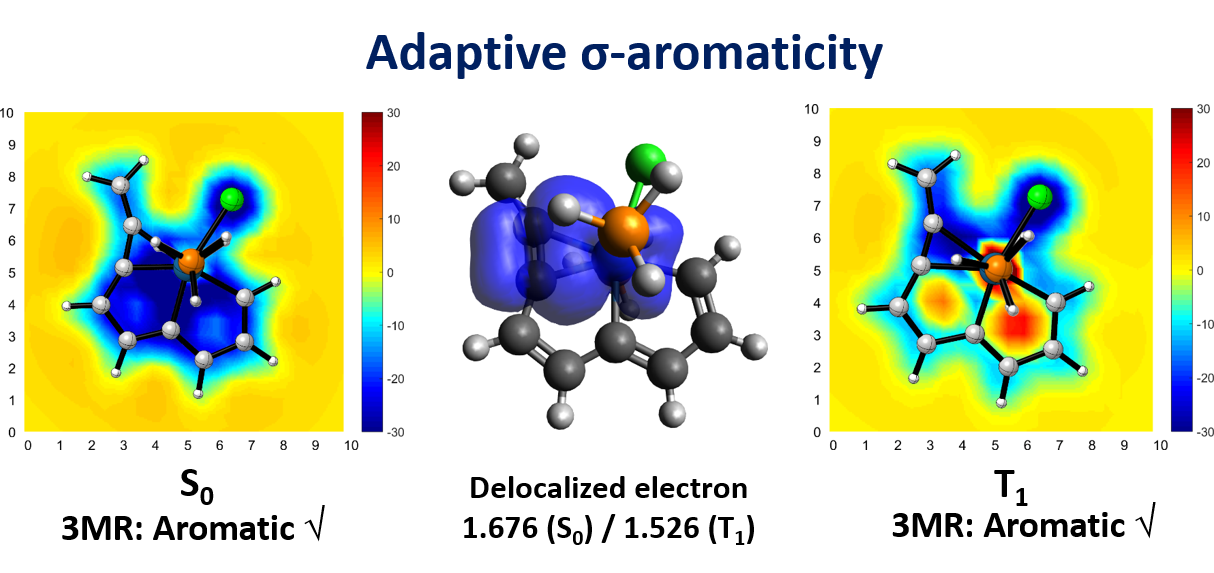
Based on Hückel’s and Baird’s rules, species are aromatic either in the lowest singlet state (S0) or the lowest triplet state (T1) only. Thus, species with adaptive aromaticity (with aromaticity in both the S0 and T1 states) is particularly rare. On the other hand, σ-aromaticity in the T1 state has been underdeveloped, let alone adaptive σ-aromaticity. Herein, via various aromaticity indices including NICS, ACID and EDDB, we demonstrate adaptive s-aromaticity in an unsaturated three-membered ring, which is a traditional area dominated by π-aromaticity.
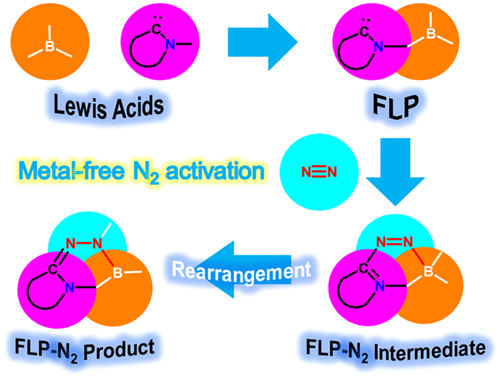
Activation of the strongest triplet bond in molecular nitrogen (N2) under mild conditions is particularly challenging. Recently, its fixation and reduction were achieved by highly reactive dicoordinated borylene species at ambient conditions, ripping the limits of harsh reaction conditions by metallic species. Less reactive species with a facile preparation could be desirable for next-generation N2 activation. Now density functional theory calculations reveal that tricoordinated boranes could be a potential candidate of N2 activation/functionalization.
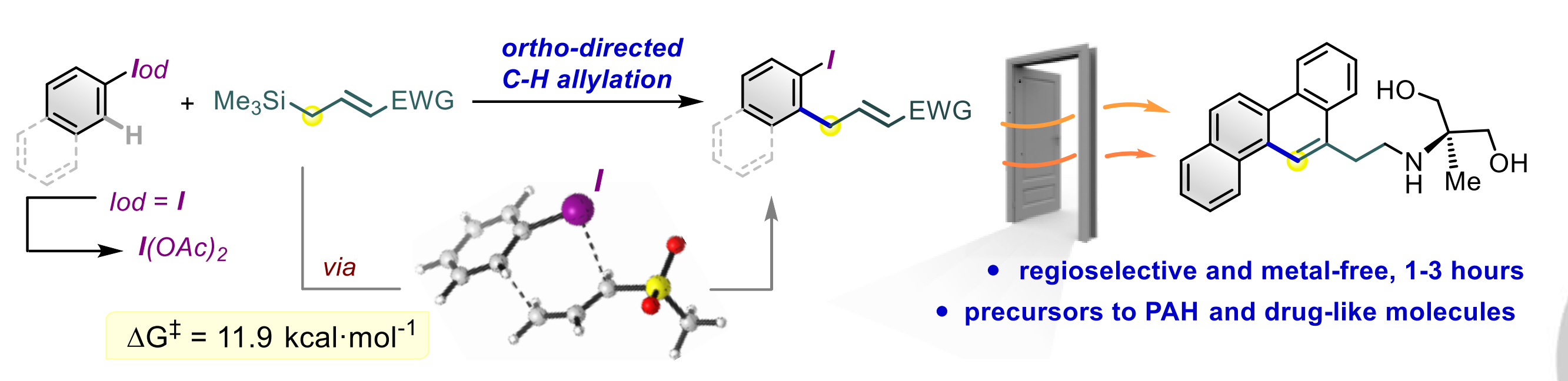
A metal‐free C‐H allylation strategy is described to access diverse functionalized ortho ‐allyl‐iodoarenes. The method employs hypervalent (diacetoxy)iodoarenes and proceeds through the iodane‐guided “iodonio‐Claisen” allyl transfer. The use of allylsilanes bearing electron‐withdrawing functional groups unlocks the functionalization of a broad range of substrates, including electron‐neutral and electron‐poor rings.
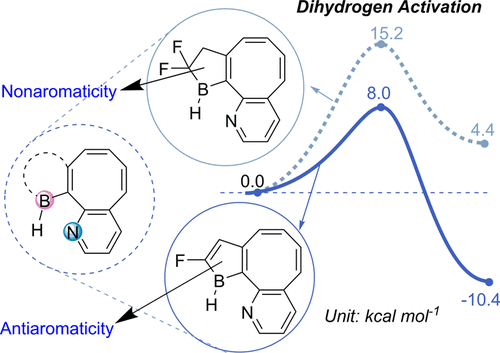
Aromaticity and frustrated Lewis pairs (FLP), two important concepts in chemistry, have attracted considerable attention from theoretical and experimental chemists. However, combining these two concepts together for H2 activation is less developed. Herein, we report a density functional theory study on antiaromaticity-promoted H2 splitting. The antiaromatic borole (as Lewis acid) and aromatic pyridine (as Lewis base) were introduced into the cyclooctetraene skeleton. Due to the geometric constraints, such systems can be classified as FLPs.
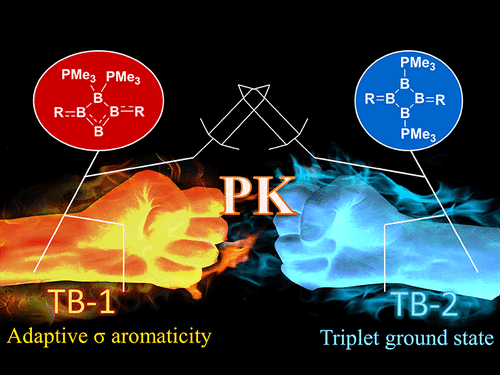
In comparison with the widely recognized π aromaticity, σ aromaticity is a less developed concept in chemistry, especially for unsaturated systems. Moreover, most studies on σ aromaticity have been mainly limited to the ground state of saturated systems; unsaturated species with σ aromaticity in the excited state have never been reported.
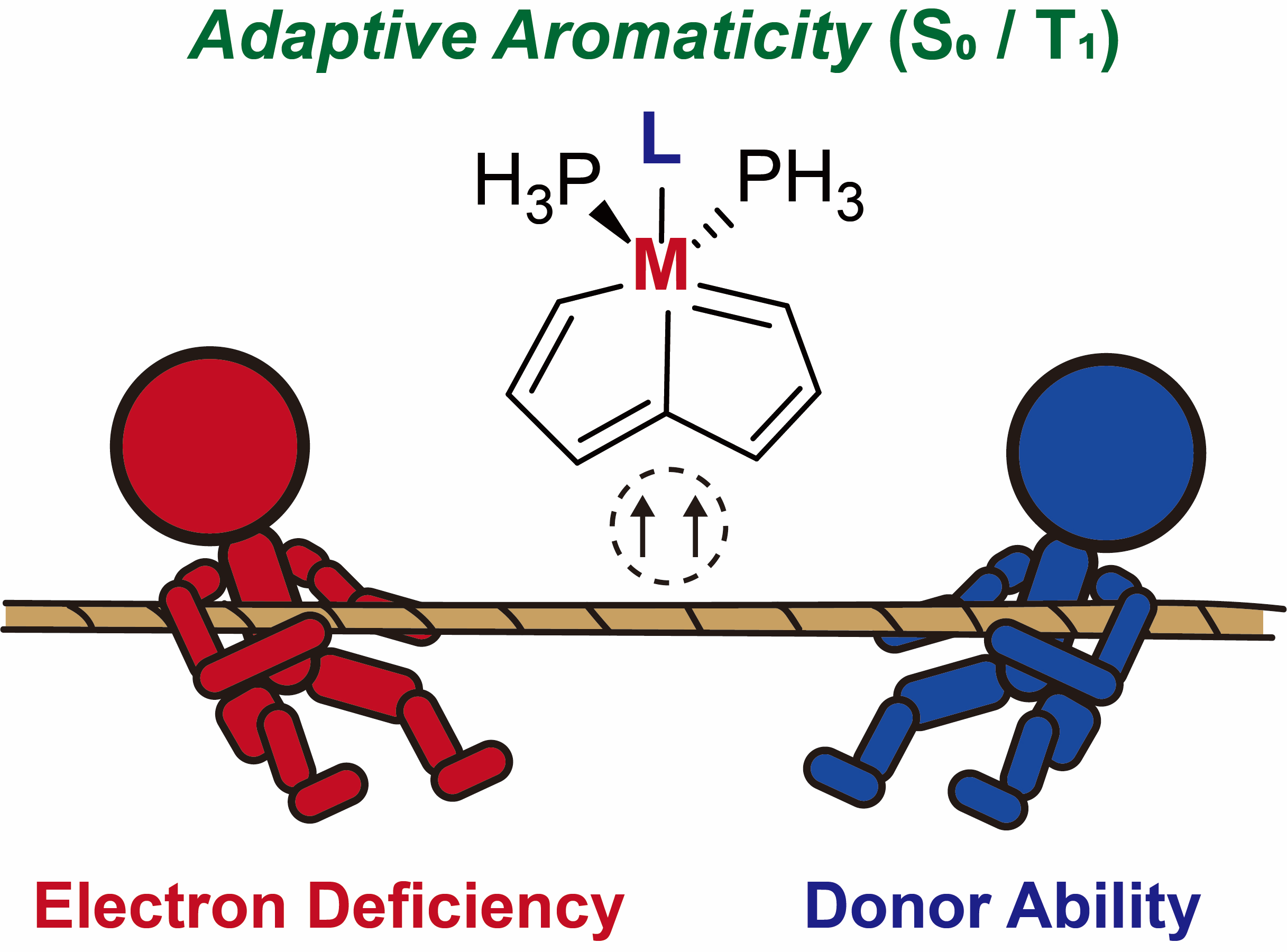
Species with adaptive aromaticity are aromatic in the ground and lowest‐lying triplet excited states and they have normally intermediate singlet‐triplet gaps. Few examples of compounds with adaptive aromaticity are known to date, including 16‐valence‐electron (16e) metallapentalenes. A sweeping search could be conducted to discover new members of this group, but efficient designs with an explicit strategy would facilitate the quest for new members of this elusive family.
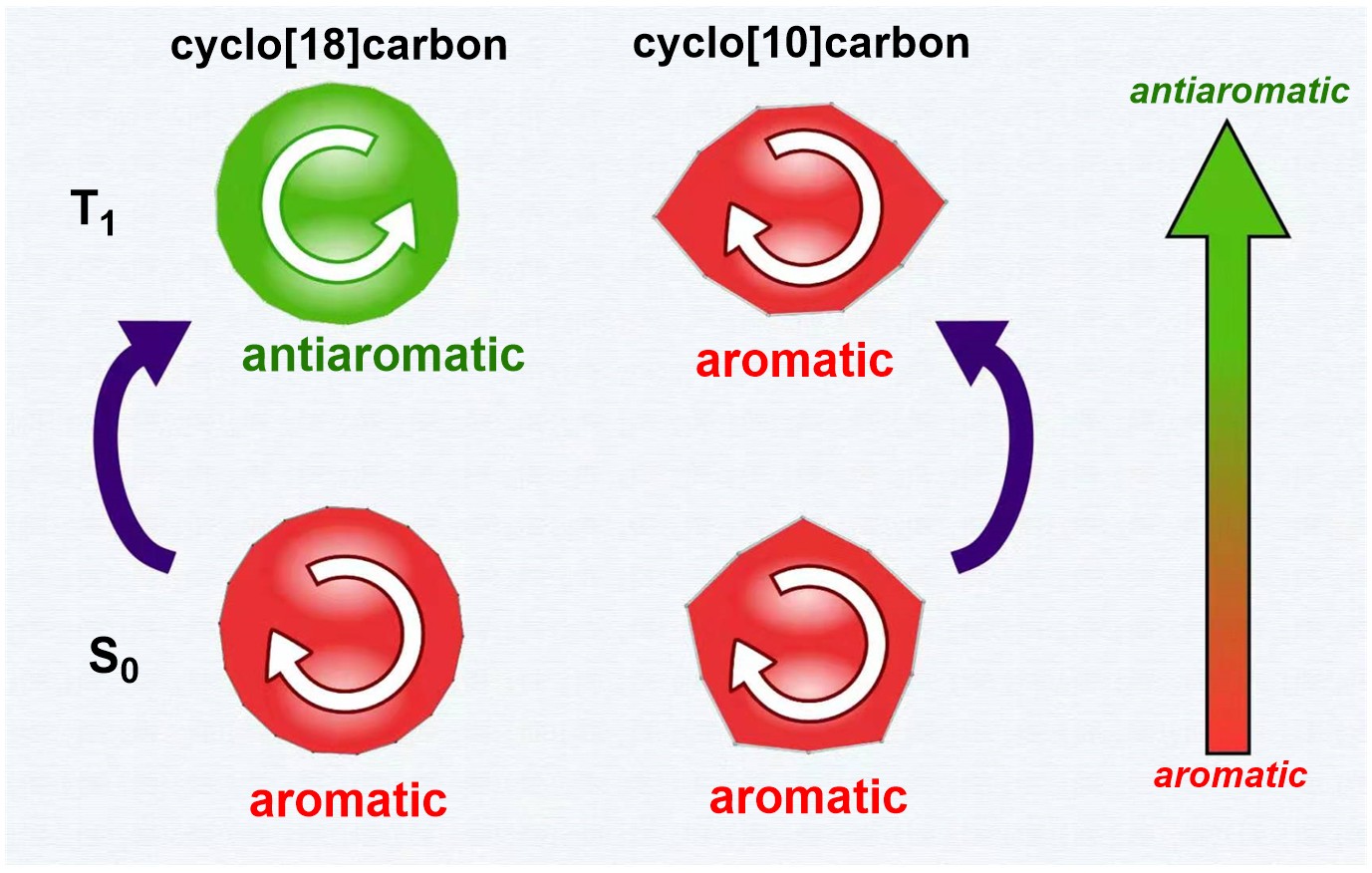
Discovery of species with adaptive aromaticity (being aromatic in both the lowest singlet and triplet states) is particularly challenging as cyclic species are generally aromatic either in the ground state or in the excited state only according to Hückel’s and Baird’s rules.
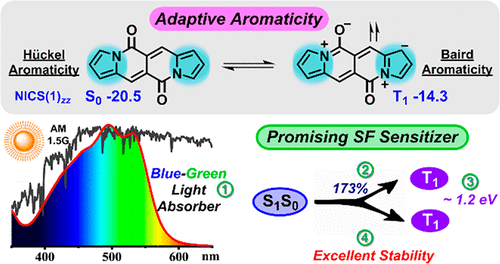
Singlet fission (SF) materials hold the potential to increase the power conversion efficiency of solar cells by reducing the thermalization of high-energy excited states. The major hurdle in realizing this potential is the limited scope of SF-active materials with high fission efficiency, suitable energy levels, and sufficient chemical stability.
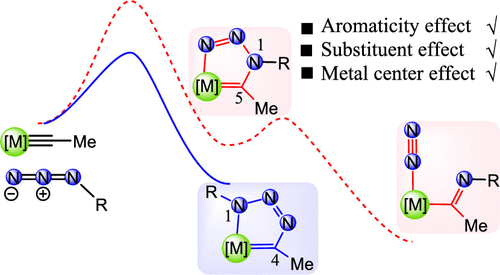
Density functional theory calculations were used to investigate the reaction mechanisms on [3 + 2] cycloaddition reactions of azides with metal carbyne complexes. Our results reveal that the formation of a 1,4-metallatriazole regioisomer is a kinetically favorable process in comparison with the formation of 1,5-metallatriazole. Aromaticity plays an important role in stabilizing the products in these reactions. Further analyses show that the electron-donating ligand on metal centers or the electron-withdrawing group on the azide could accelerate the [3 + 2] cycloaddition reaction.
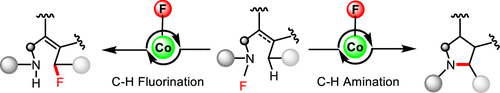
A redox neutral radical-relay cobalt-catalyzed intramolecular C–H fluorination of N-fluoroamides featuring the in situ formed cobalt fluorides as the latent radical fluorinating agents is reported. Moreover, the reactivity of such a cobalt catalysis could be diverted from C–H fluorination to amination by engineering substrates’ conformational flexibility. Preliminary mechanistic studies (UV–vis spectroscopy, cyclic voltammetry studies and DFT calculations, etc.) support the reaction proceeding a redox neutral radical-relay mechanism.
Copyright © 2025,
Theme Originally Created by Devsaran
