Probing the origin of adaptive aromaticity in 16‐valence‐electron metallapentalenes
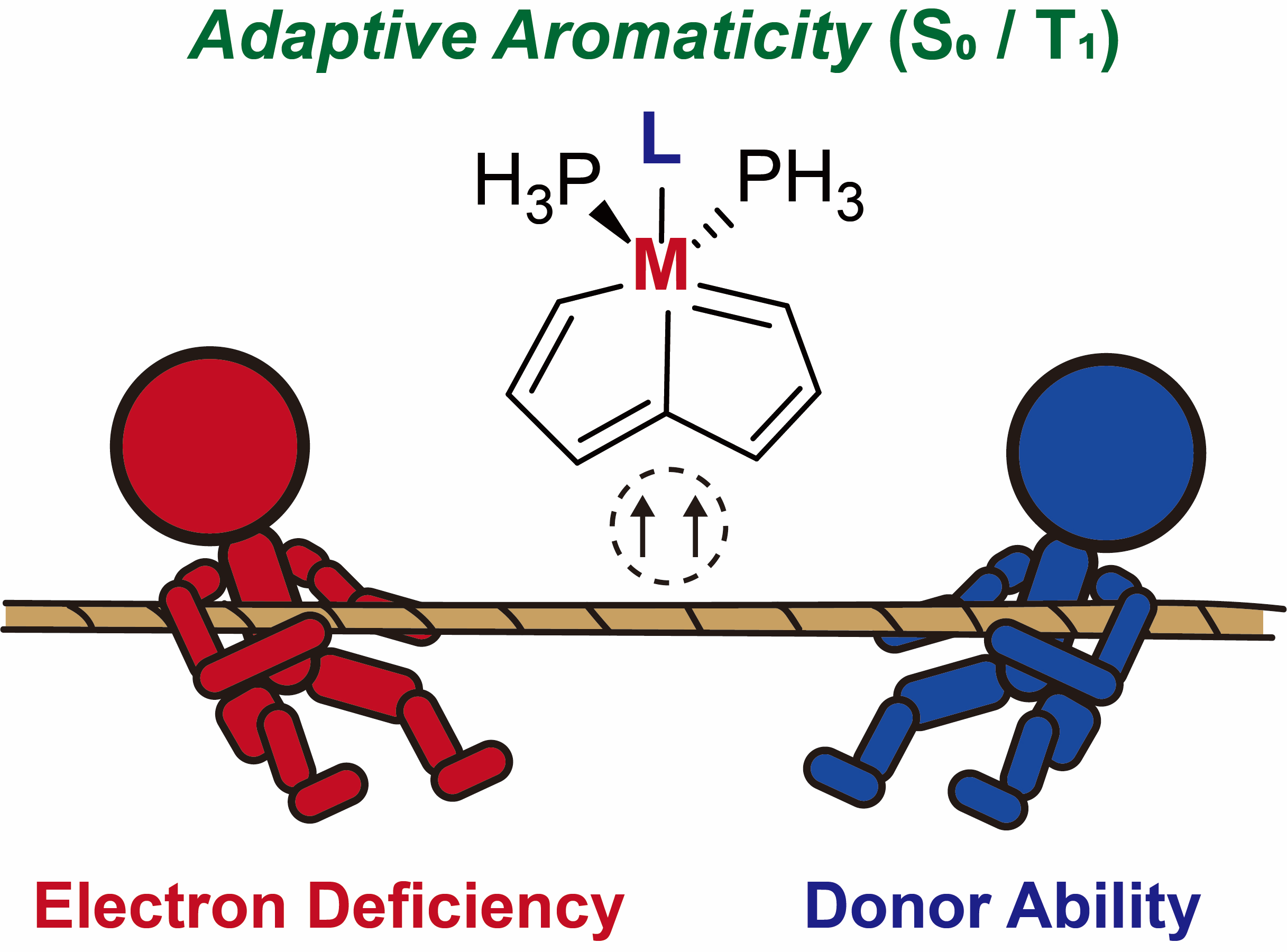
Species with adaptive aromaticity are aromatic in the ground and lowest‐lying triplet excited states and they have normally intermediate singlet‐triplet gaps. Few examples of compounds with adaptive aromaticity are known to date, including 16‐valence‐electron (16e) metallapentalenes. A sweeping search could be conducted to discover new members of this group, but efficient designs with an explicit strategy would facilitate the quest for new members of this elusive family. Density functional theory calculations and aromaticity evaluations have been performed to reveal the nature of triplet‐state aromaticity in 16e metallapentalenes. Our results show that coordination of strong σ‐ or π‐donor ligands helps achieving adaptive aromaticity of 16e metallapentalenes by means of a spin delocalization mechanism. These results have important implications for understanding the unusual properties of the organometallic adaptive aromatics, leading the way to efficient design of new compounds with tunable singlet‐triplet gaps.
