Nature Chemistry


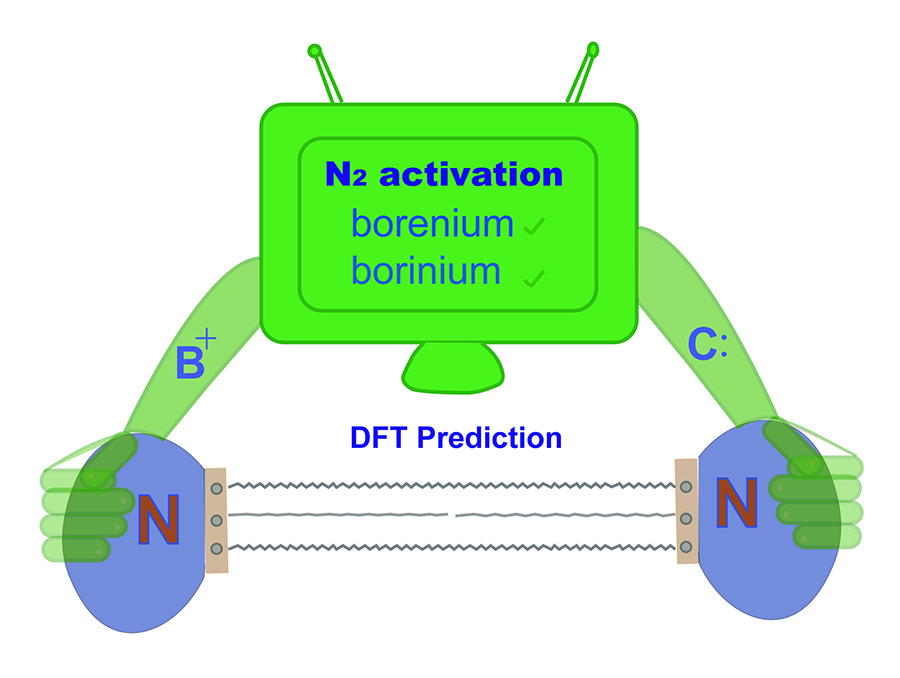
Activation of thermodynamically stable and kinetically inert dinitrogen (N2) has been a great challenge due to a significantly strong triple bond. Recently, the experimental study on the N2 activation by boron species, a highly reactive two-coordinated borylene, broke through the limitation of traditional strategy of N2 activation by metal species. Still, the study on metal-free N2 activation remains undeveloped.
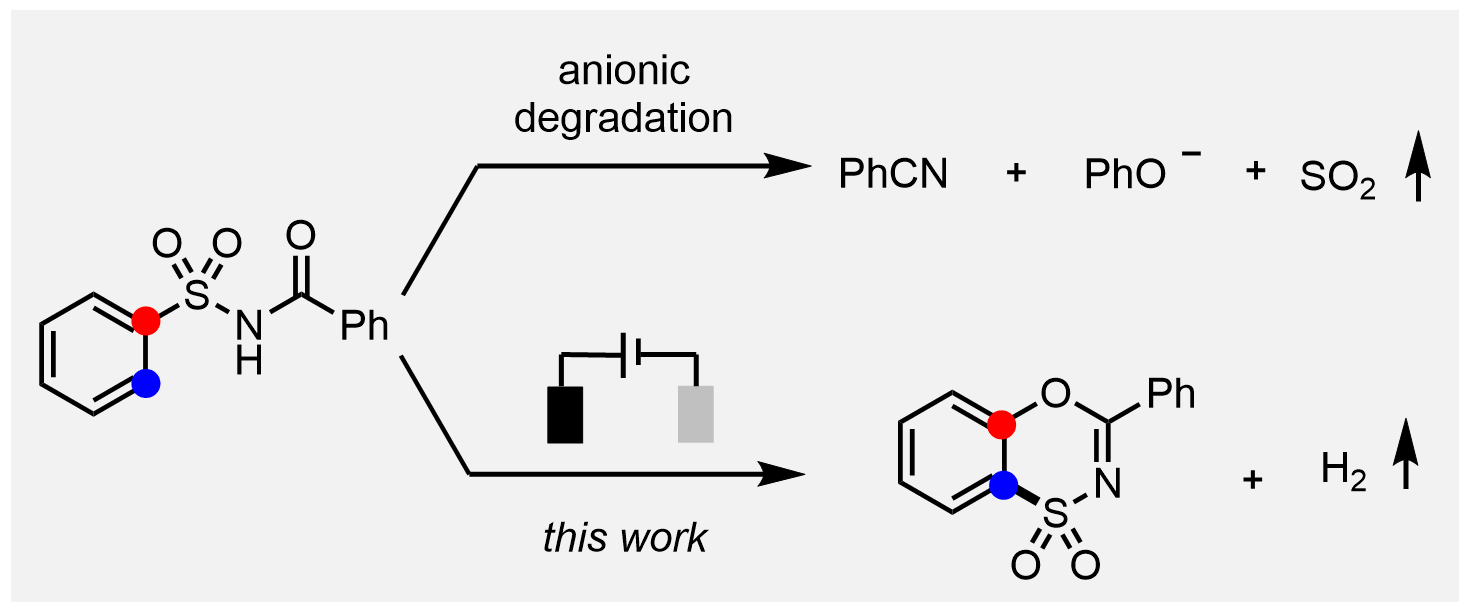
Benzoxathiazine dioxide, as a bioisostere of the clinically widely used diazoxide, exhibits interesting biological activity. However, limited success has been achieved in terms of its concise and direct synthesis. We report herein a facile electrochemical migratory cyclization of N -acylsulfonamides to access a diverse array of benzoxathiazine dioxides. The inclusion of electrochemistry is crucial for realizing such a novel transformation, which is substantiated both by the experiments and density-functional-theory calculations.
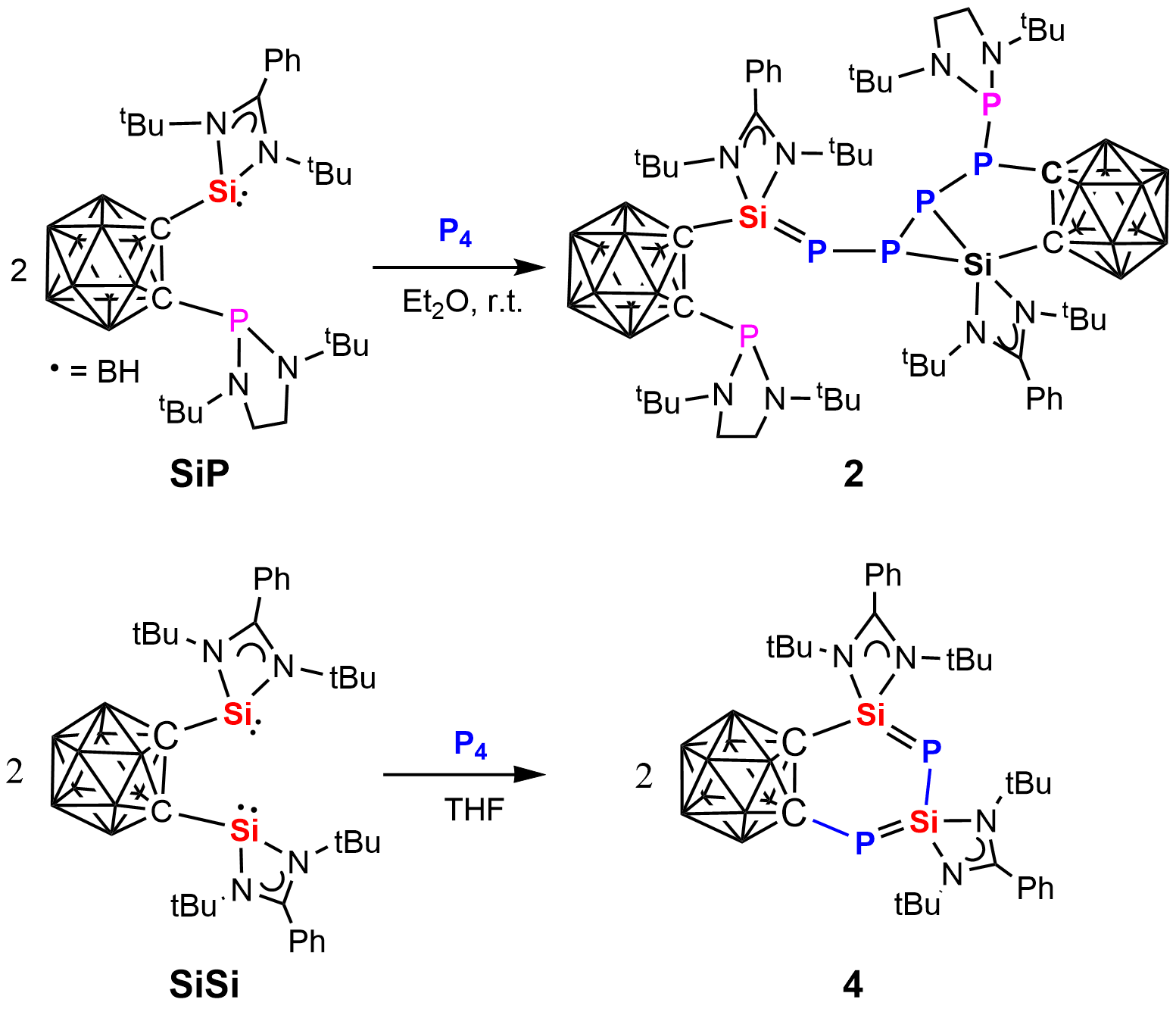
New types of metal-free white phosphorus (P4) activation are reported. While the phosphine-silylene-substituted dicarborane 1, CB-SiP {CB = ortho-C,C´-C2B10H10, Si = PhC(tBuN)2Si, P = P[N(tBu)CH2]2}, activates white phosphorus in a 2:1 molar ratio to yield the P5-chain containing species 2, the analogous bis(silylene)-substituted compound 3, CB-Si2, reacts with P4 in the molar ratio of 2:1 to furnish the first isolable 1,3-diphospha-2,4-disilabutadiene (Si=P-Si=P-containing) compound 4.
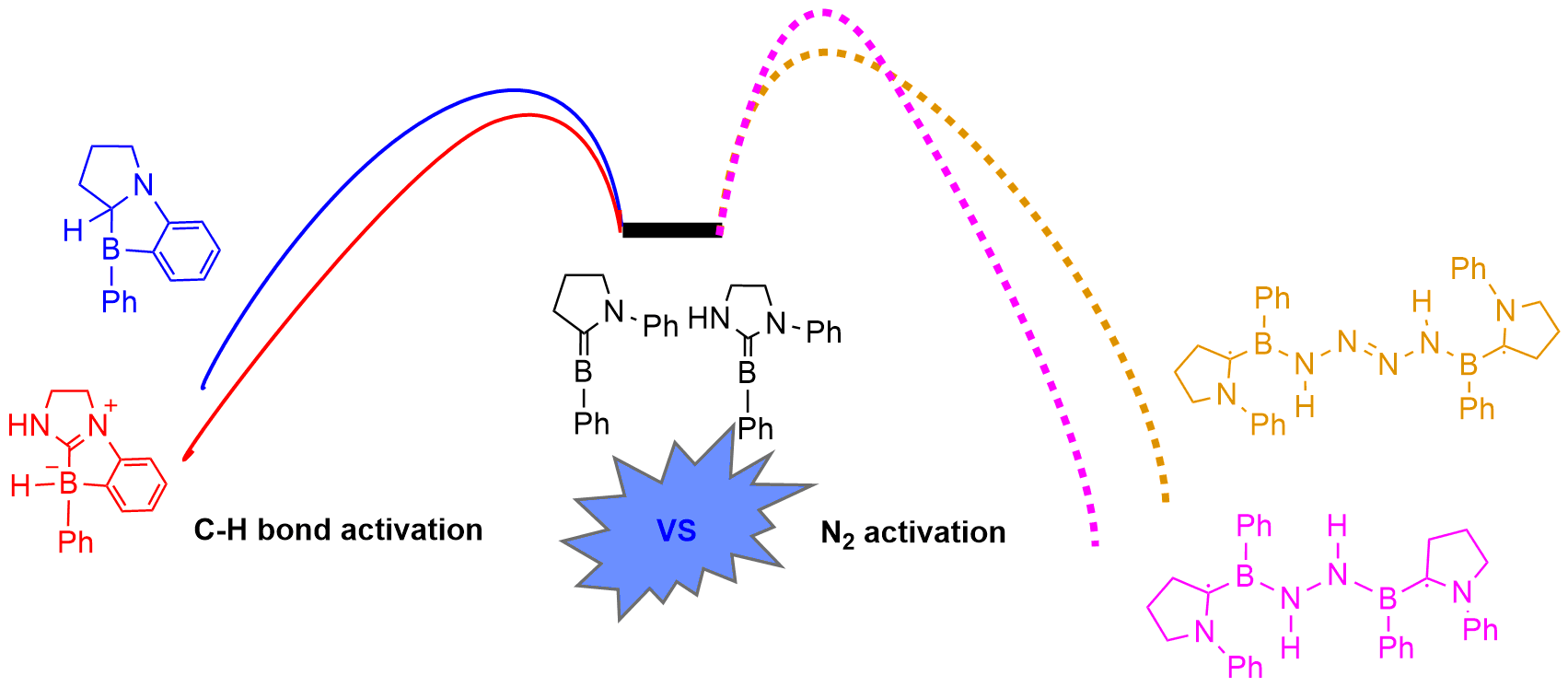
Dinitrogen (N2) activation is particularly challenging due to the significantly strong N≡N bond, let alone the catenation of two N2 molecules. Recent experimental study shows that cyclic (alkyl)(amino)carbene (CAAC)-stabilized borylenes are able to tackle N2 activation and coupling below room temperature. Here we carry out density functional theory calculations to explore the corresponding reaction mechanisms. The results indicate that the reaction barrier for the dinitrogen activation by the first borylene is slightly higher than that by the second borylene.
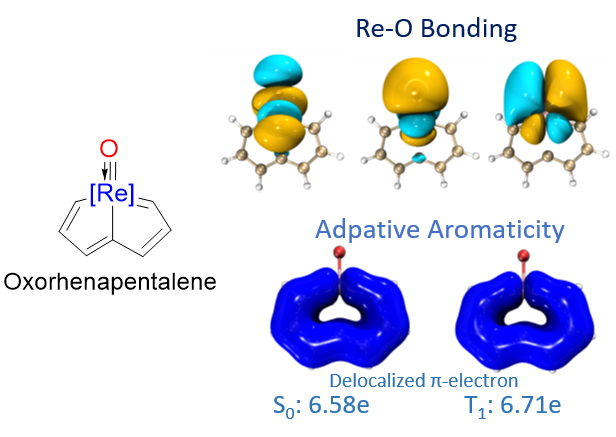
In general, species could be aromatic in the ground or excited state only according to Hückel’s and Baird’s rules. Thus, adaptive aromatics are particularly rare as they can be aromatic in both the lowest singlet and triplet states (S0 and T1). Here, we carry out density functional theory calculations on a series of rhenium oxo complexes to examine their structure, bonding, and aromaticity. It is found that all these complexes are aromatic in the S0 state. In contrast, their T1 states could be antiaromatic, nonaromatic, or aromatic depending on the ligands.
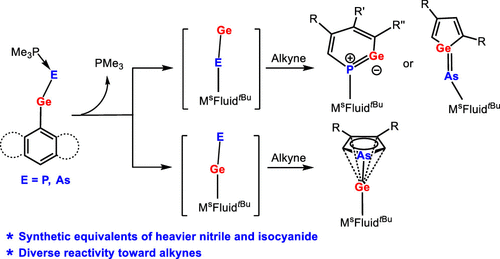
The reactions of chlorogermylene MsFluindtBu-GeCl 1, supported by a sterically encumbered hydrindacene ligand MsFluindtBu, with NaPCO(dioxane)2.5 and NaAsCO(18-c-6) in the presence of trimethylphosphine afforded trimethylphosphine-stabilized germylidenyl-phosphinidene 2 and -arsinidene 3, respectively. Structural and computational investigations reveal that the Ge–E′ bond (E′ = P and As) features a multiple-bond character.
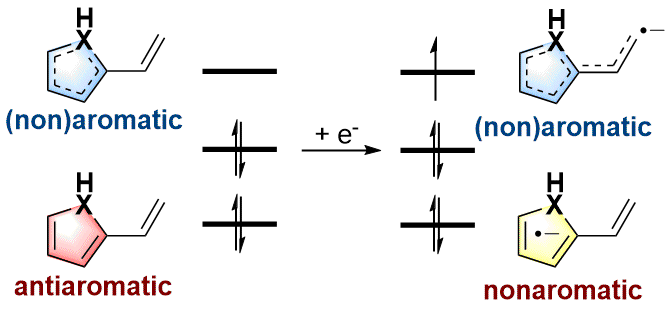
As an electron-rich species, radical anions have a wide range of applications in organic synthesis. In addition, aromaticity is an essential concept in chemistry that has attracted considerable attention from experimentalists and theoreticians. However, it remains unknown whether there is a relationship between aromaticity and thermodynamic stability of a radical anion. In this work, we demonstrate that the thermodynamically stable radical anions could be formed by the corresponding antiaromatic neutral species through density functional theory calculations.
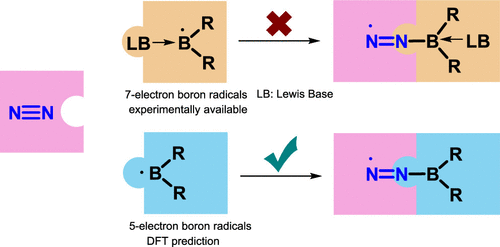
Due to the high bond dissociation energy (945 kJ mol–1) and the large highest occupied molecular orbital–lowest unoccupied molecular orbital (HOMO–LUMO) gap (10.8 eV), dinitrogen activation under mild conditions is extremely challenging. On the other hand, the conventional Haber–Bosch ammonia synthesis under harsh conditions consumes more than 1% of the world’s annual energy supply. Thus, it is important and urgent to develop an alternative approach for dinitrogen activation under mild conditions.
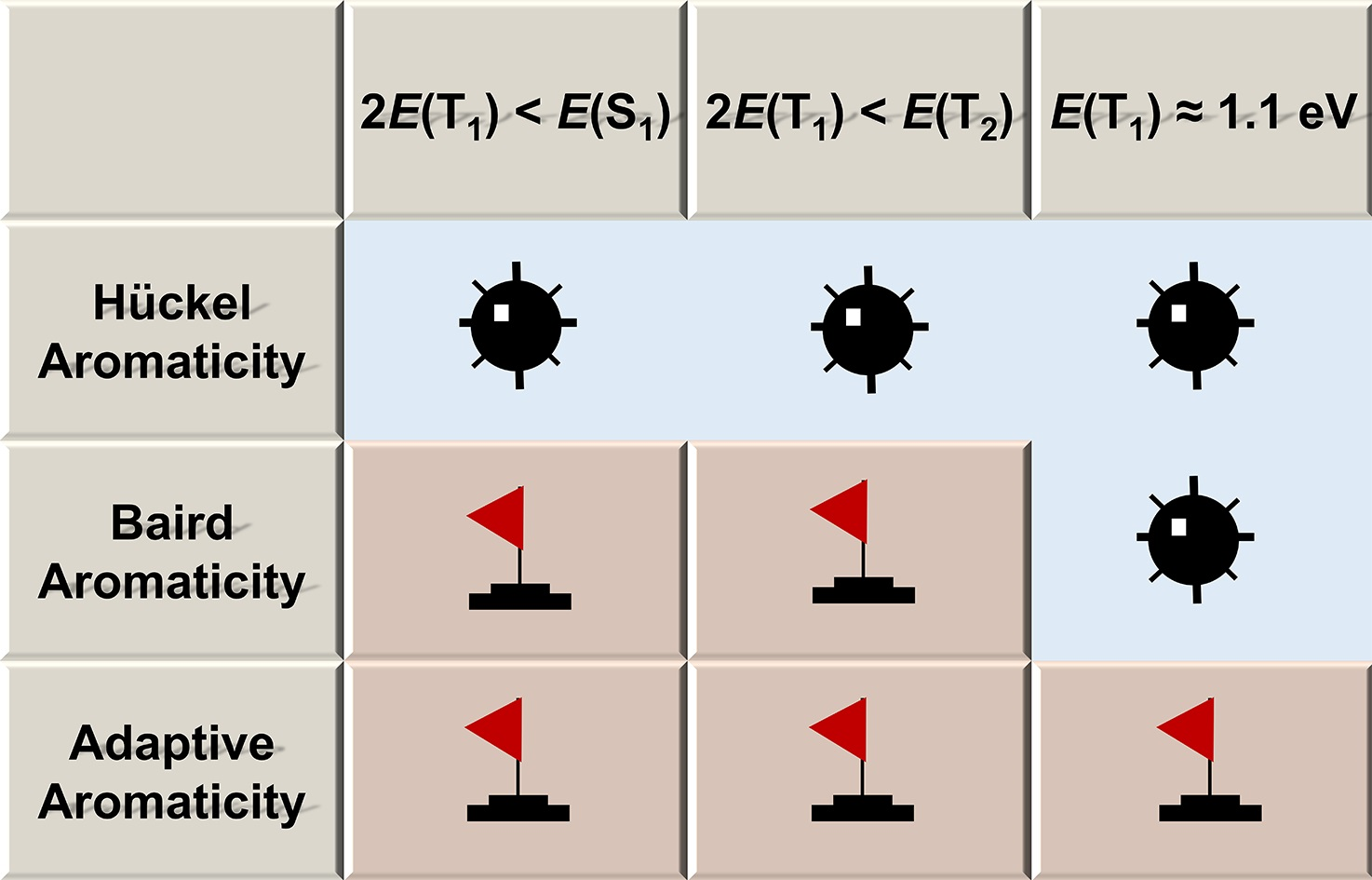
Singlet fission has attracted extensive attention from experimentalists and theoreticians due to its ability to improve photovoltaic conversion efficiency. Still, designing singlet fission materials remains challenging. In this work, we explored the relationship between adaptive aromaticity and singlet fission potentials by computationally screening the adaptive aromatic species reported by our group.
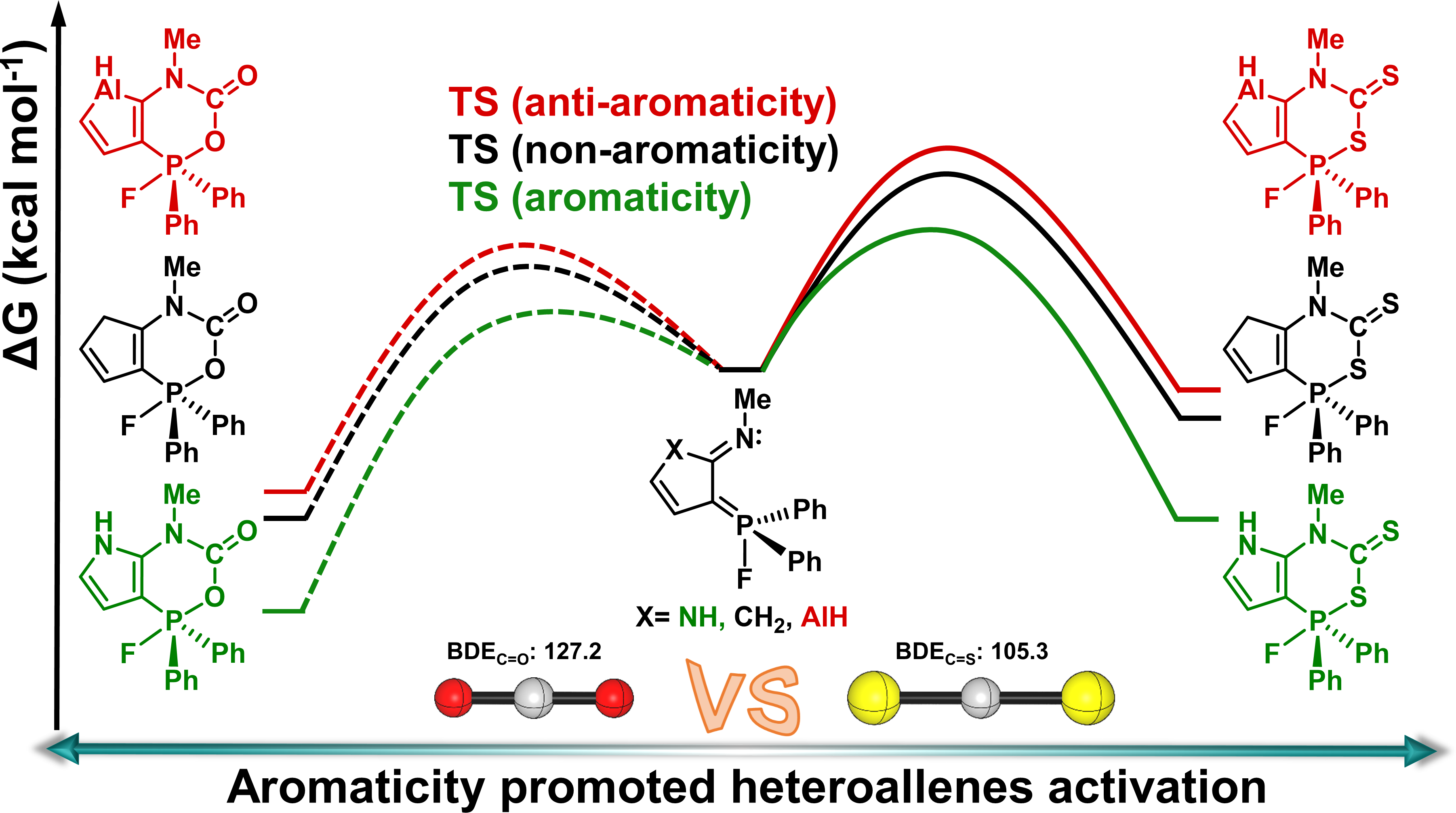
Carbon dioxide (CO2) capture has attracted considerable attention from both experimental and theoretical chemists. In comparison, Carbon disulfide (CS2) activation is less developed. Here, we carry out a thorough comparative density functional theory study to examine the reaction mechanisms of CS2 activation by five-membered heterocycles-bridged P/N frustrated Lewis pairs (FLPs).
Copyright © 2025,
Theme Originally Created by Devsaran
