Nature Chemistry


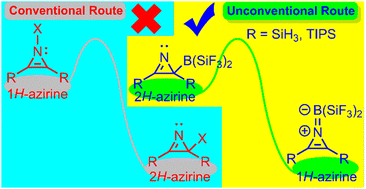
1H-azirine, a highly reactive, antiaromatic, and unstable tautomer of the aromatic, stable, and (sometimes) isolable 2H-azirine, is stabilized, both thermodynamically and kinetically, via an unprecedented route, where the latter serves as the precursor–exploiting electronic and steric elements. Our density functional theory results invite experimentalists to realize isolable 1H-azirine.
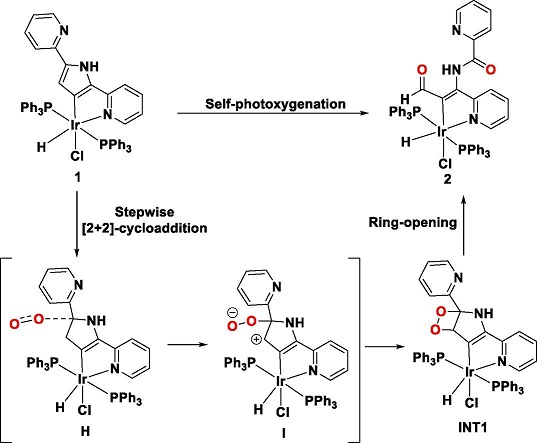
Visible-light-mediated dearomatisation of pyrroles is a powerful strategy for the synthesis of pharmaceuticals and bioactive compounds. Herein we present the chemiluminescent reaction between pyrrole metalated-Ir(III) complex [Ir(K2C,N-DPP)(H)(Cl)(PPh3)2] (1) and singlet oxygen to form a ketoamide complex [Ir(K2C,N-ketoamide)(H)(Cl)(PPh3)2] (2). Complex 2 are fully characterized by NMR and single crystal X-ray diffraction analysis.
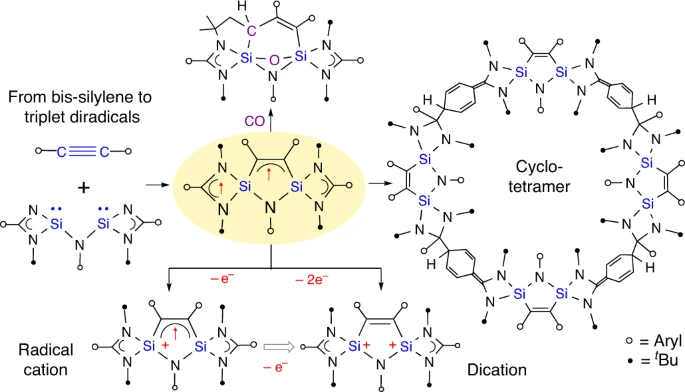
Open-shell molecules with unpaired electrons and a high-spin S ≥ 1 configuration are of fundamental importance in chemistry, biology and molecular electronics. Among metal-free systems, carbon- and silicon-based triplet diradicals with two unpaired electrons and strong ferromagnetic coupling are proposed as key intermediates in many organic and organometallic transformations but their isolation remains challenging due to their very high reactivity. Here we report the facile synthesis of isolable 1,3-disilapyrroles which act as organosilicon-based delocalized triplet diradicals.
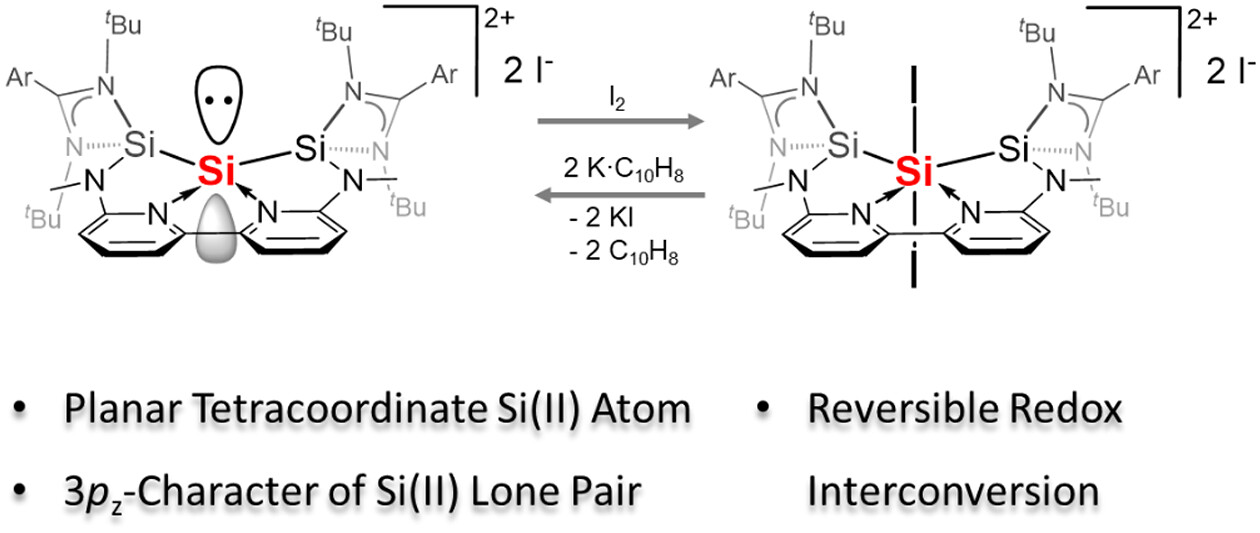
For a long time, planar tetracoordinate carbon (ptC) represented an exotic coordination mode in organic and organometallic chemistry, but it is now a useful synthetic building block. In contrast, realization of planar tetracoordinate silicon (ptSi), a heavier analogue of ptC, is still challenging. Herein we report the successful synthesis and unusual reactivity of the first ptSi species of divalent silicon present in 3, supported by the chelating bis(N-heterocyclic silylene)bipyridine ligand, 2,2′-{[(4-tBuPh)C(NtBu)]2SiNMe}2(C5N)2, 1].
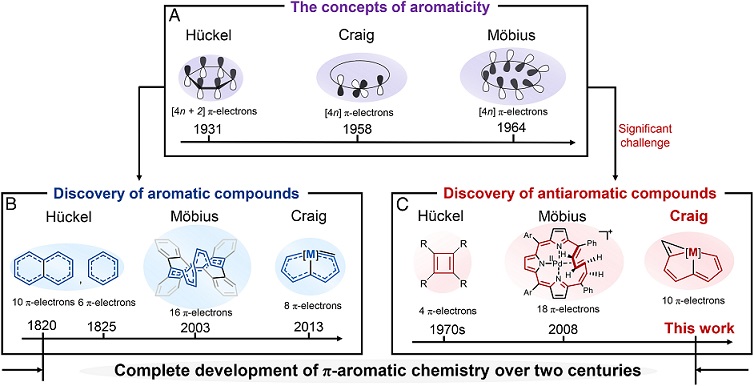
Antiaromaticity is extended from aromaticity as a complement to describe the unsaturated cyclic molecules with antiaromatic destabilization. To prepare antiaromatic species is a particularly challenging goal in synthetic chemistry because of the thermodynamic instability of such molecules. Among that, both Hückel and Möbius antiaromatic species have been reported, whereas the Craig one has not been realized to date. Here, we report the first example of planar Craig antiaromatic species.
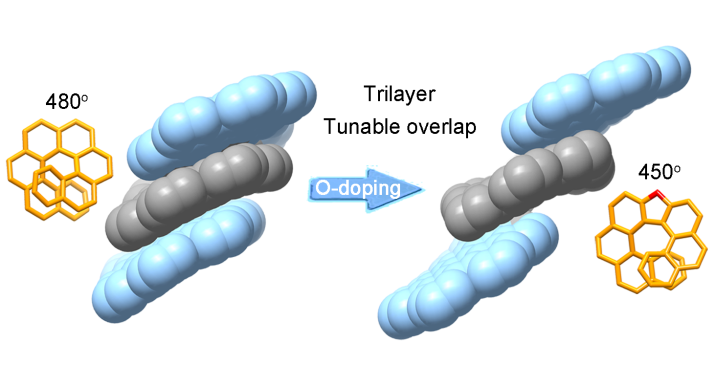
The synthesis of well-defined nanocarbon multilayers, beyond the bilayer structure, is still a challenging goal. Herein, two trilayer nanographenes were synthesized by covalently linking nanographene layers through helicene bridges. The structural characterization of the trilayer nanographenes revealed a compact trilayer-stacked architecture.
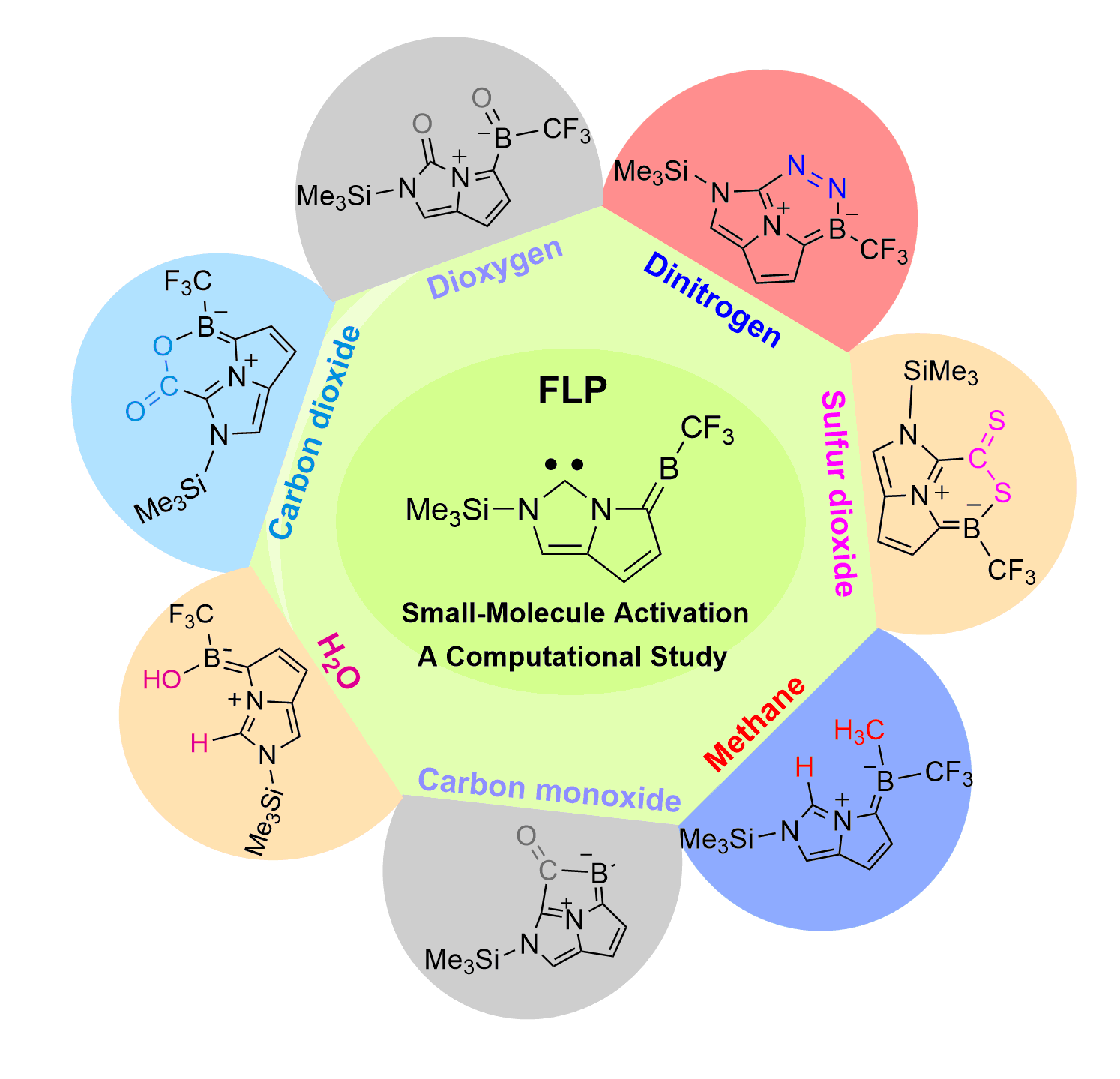
Dinitrogen (N2) activation is particularly challenging under ambient conditions because of its large highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) gap (10.8 eV) and high bond dissociation energy (945 kJ mol–1) of the NΞN triple bond, attracting considerable attention from both experimental and theoretical chemists. However, most effort has focused on metallic systems. In contrast, nitrogen activation by frustrated Lewis pairs (FLPs) has been initiated recently via theoretical calculations.
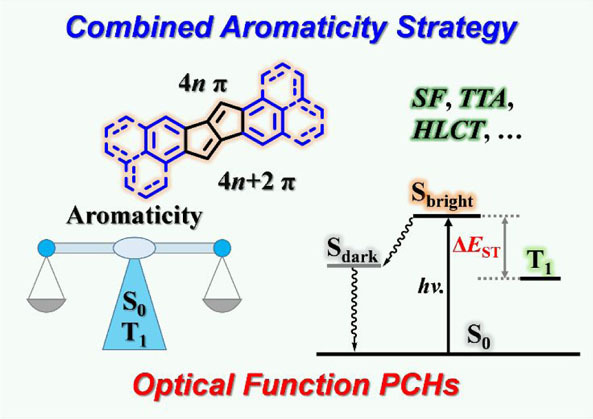
Understanding the structure–property relationships in polycyclic conjugated hydrocarbons (PCHs) is crucial for controlling their electronic properties and developing new optical function materials. Aromaticity is a fundamentally important and intriguing property of numerous organic chemical structures and has stimulated a myriad of experimental and theoretical investigations. Exploiting aromaticity rules for the rational design of optoelectronic materials with the desired photophysical characteristics is a challenging yet fascinating task.
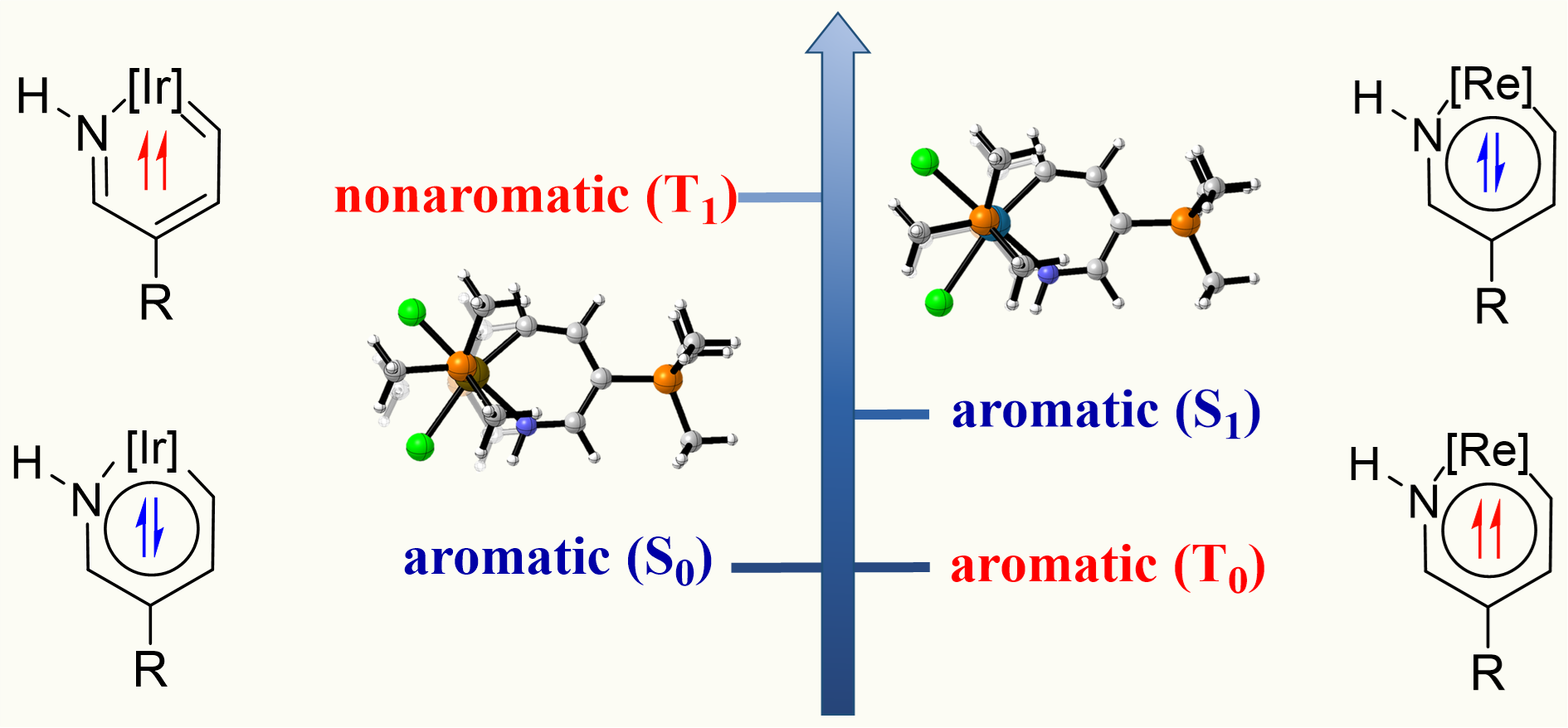
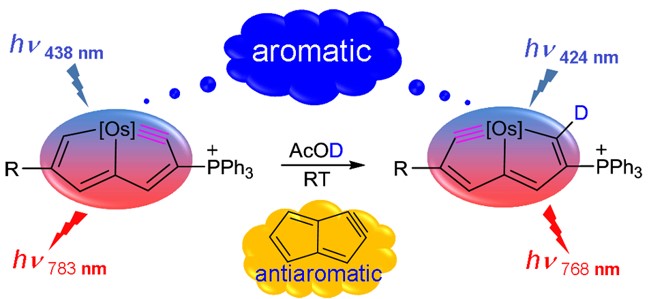
In general, compounds exhibit one-state aromaticity in either the ground or excited state according to the Hückel’s and Baird’s rules. Thus, species with two-state aromaticity in the lowest singlet and triplet states (termed as adaptive aromaticity) are rare. Understanding the underlying mechanism for achieving adaptive aromaticity is important to enrich this rare family. Here we carry out density functional theory (DFT) calculations to probe the origin of adaptive aromaticity in metallapyridiniums.
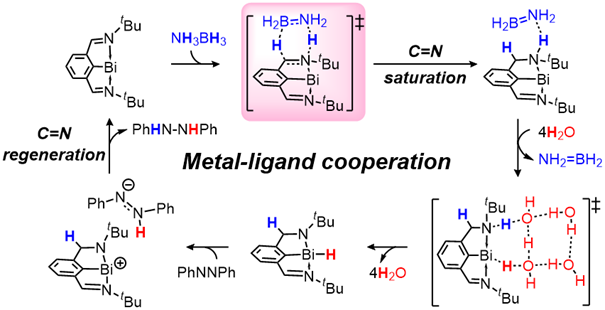
Transfer hydrogenation of azobenzene with ammonia borane mediated by pincer bismuth complex 1 were systematically investigated through density functional theory calculations. An unusual metal-ligand cooperation mechanism was disclosed, in which the saturation/regeneration of the C=N functional group on the pincer ligand plays an essential role. The reaction is initiated by the hydrogenation of the C=N bond (saturation) with ammonia borane to afford 3CN, which is the rate-determining step with Gibbs energy barrier (ΔG≠) and Gibbs reaction energy (ΔG) of 25.6 and -7.3 kcal/mol, respectively.
Copyright © 2025,
Theme Originally Created by Devsaran
