Probing the Mechanism of Adaptive Aromaticity in Metallapyridiniums
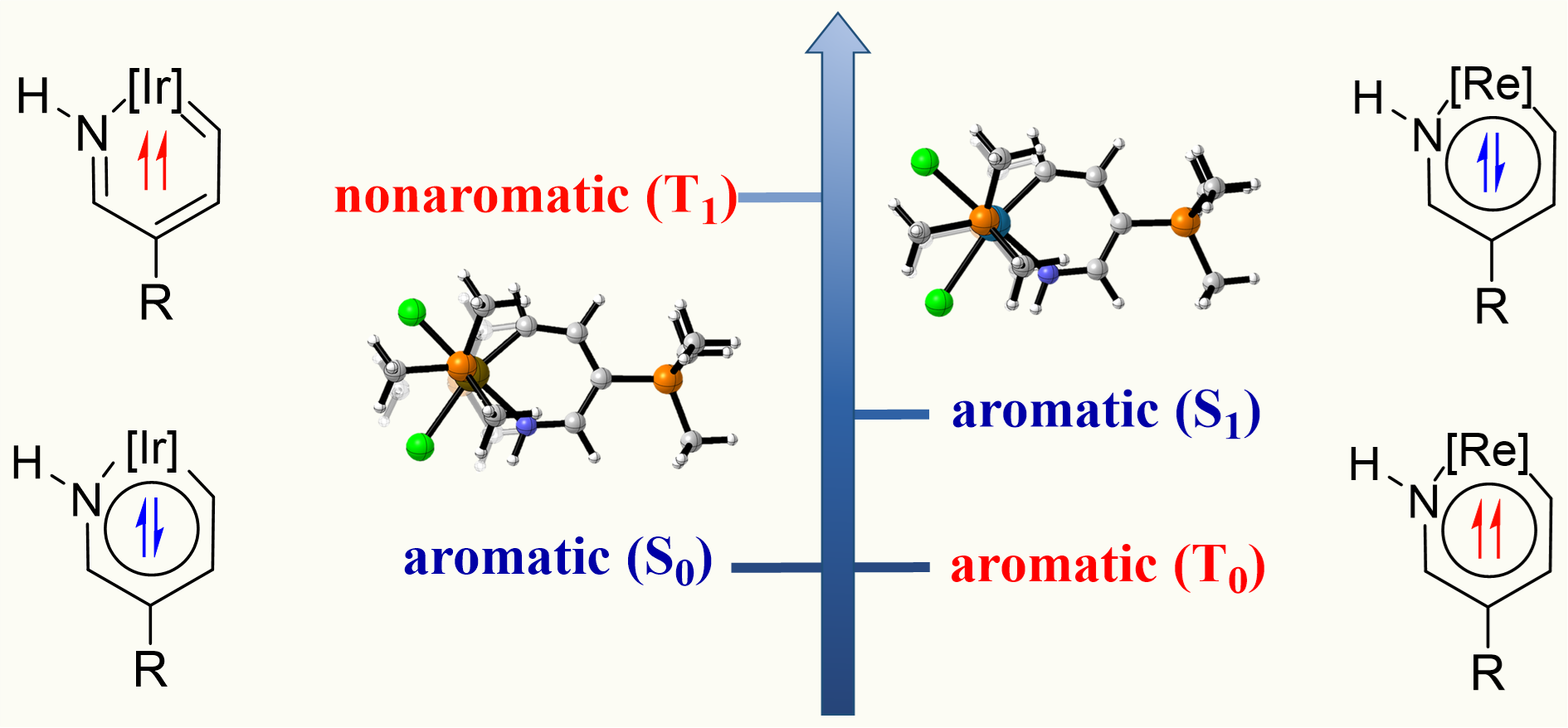
In general, compounds exhibit one-state aromaticity in either the ground or excited state according to the Hückel’s and Baird’s rules. Thus, species with two-state aromaticity in the lowest singlet and triplet states (termed as adaptive aromaticity) are rare. Understanding the underlying mechanism for achieving adaptive aromaticity is important to enrich this rare family. Here we carry out density functional theory (DFT) calculations to probe the origin of adaptive aromaticity in metallapyridiniums. Specifically, rhenapyridiniums and osmapyridiniums both exhibit adaptive aromaticity whereas iridapyridiniums do not. Further analysis reveals that the strength of metal-carbon and metal-nitrogen bonds in metallapyridiniums is the key factor to achieve aromaticity in the lowest triplet state. Blocking the spin delocalization in the six-membered ring of metallapyridiniums also help the persistence of aromaticity in the lowest triplet state. Ligand effects on the adaptive aromaticity in metallapyridiniums are also examined. Our findings not only expand the scope of the concept of adaptive aromaticty but also provide an explicit strategy for achieving adaptive aromaticity in metallapyridiniums.
https://pubs.rsc.org/en/content/articlelanding/2023/qi/d2qi02119f
