Nature Chemistry
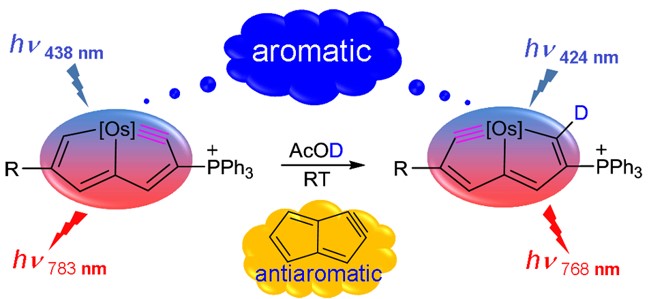


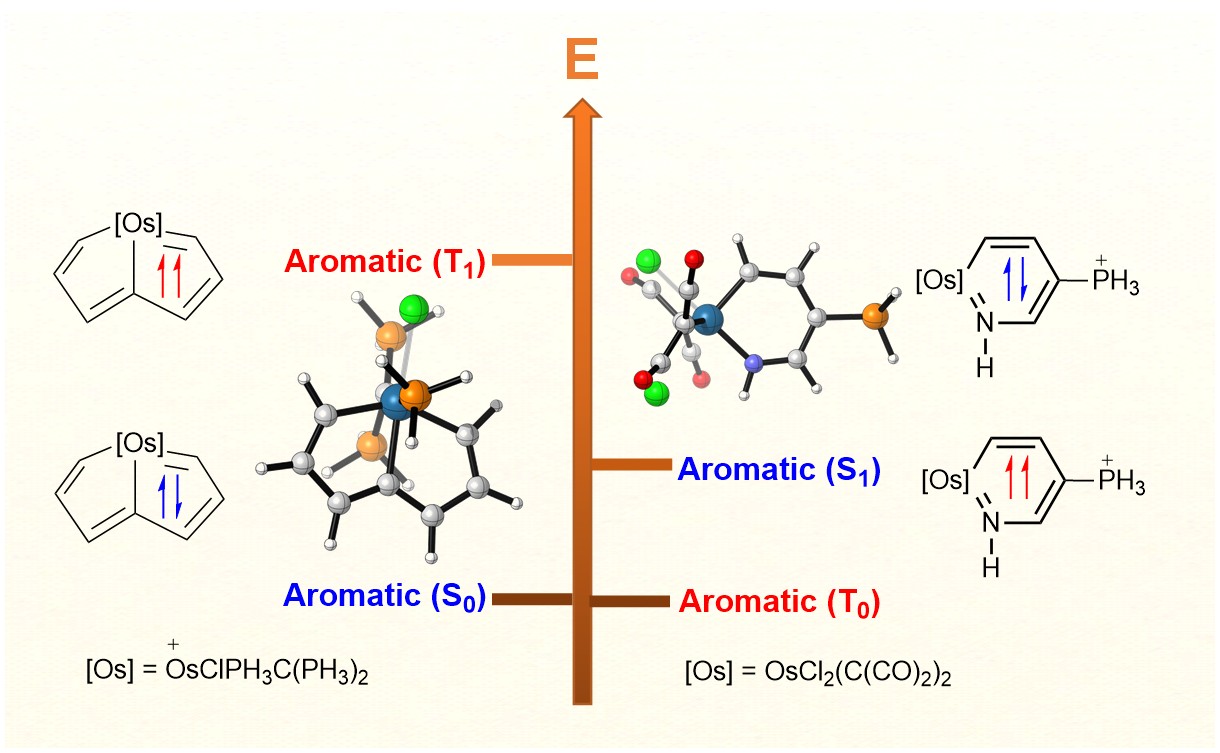
Discovery of species with adaptive aromaticity, being aromatic in both the lowest-lying singlet and triplet states (S0 and T1), is a significant challenge because cyclic conjugated complexes are commonly aromatic in one state only according to Hückel’s and Baird’s rules. On the other hand, the carbone ligands containing two lone pair electrons at the carbon(0) atom have attracted considerable attention from both theoretical and experimental chemists recently.
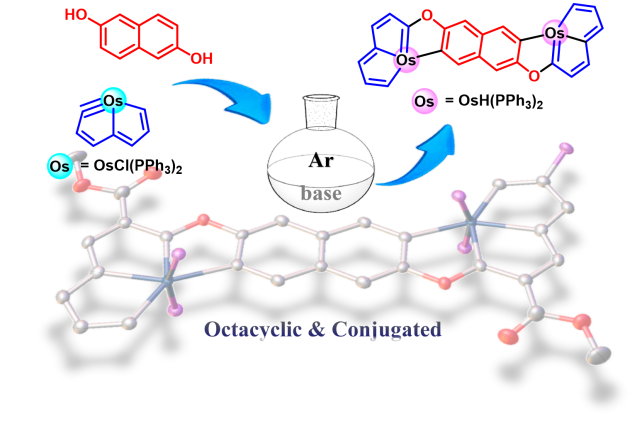
Metallaaromatics as a new class of organometallic compounds have attracted considerable attention in recent years. Metallaaromatic compounds where the number of fused rings forming the metalla-polycyclic skeleton exceeds seven have not been reported to date. Likewise, metallaaromatic compounds containing two transition-metal centres are rarely encountered in the literature. In this work two dicationic diosma-octacyclic complexes have successfully been synthesized and fully characterized.
Aromaticity and hyperconjugation are two fundamental concepts in chemistry. Combining them together led to the proposal of the concept of hyperconjugative aromaticity by Mulliken in 1939. Now, it has been attracting considerable attention from both theoretical and experimental chemists. Recently, the concept of hyperconjugative aromaticity has been extended from main-group substituents to transition metal systems including groups 9, 10, and 11 transition metal substituents.
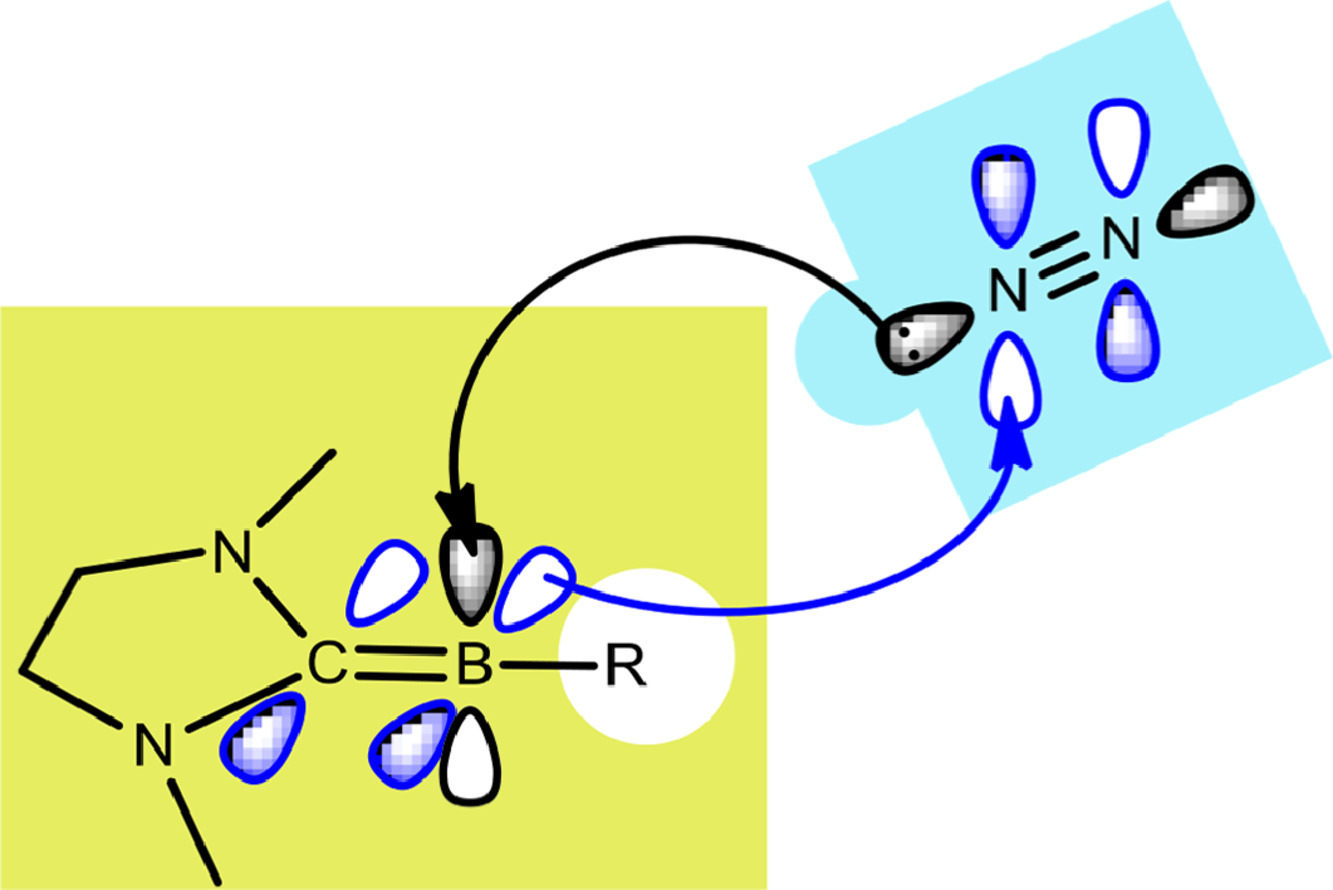
Dinitrogen activation under mild conditions is important but extremely challenging due to the inert nature of the N-N triple bond evidenced by high bond dissociation energy (945 kJ/mol) and large HOMO-LUMO gap (10.8 eV). In comparison with largely developed transition metal systems, the reported main group species on dinitrogen activation are rare. Here, we carry out density functional theory calculations on methyleneboranes to understand the reaction mechanisms of their dinitrogen activation.
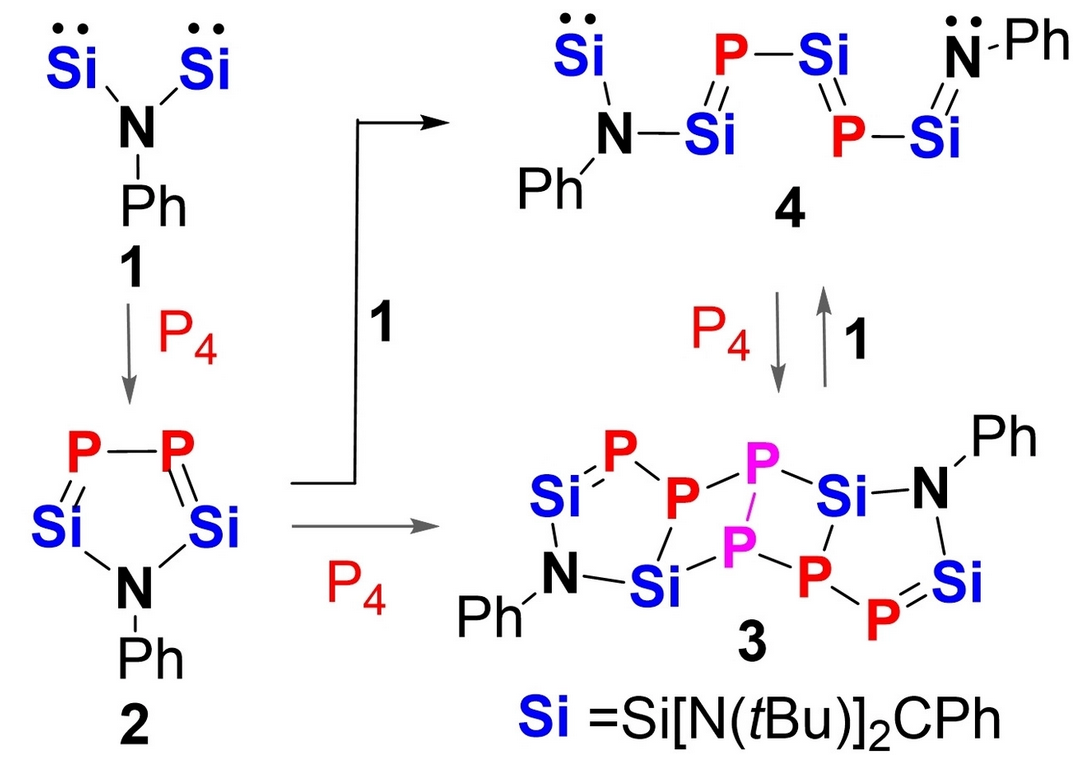
White phosphorus (P4) undergoes degradation to P2 moieties if exposed to the new N,N-bis(silylenyl)aniline PhNSi2 1 (Si=Si[N(tBu)]2CPh), furnishing the first isolable 2,5-disila-3,4-diphosphapyrrole 2 and the two novel functionalized Si=P doubly bonded compounds 3 and 4. The pathways for the transformation of the non-aromatic 2,5-disila-3,4-diphosphapyrrole PhNSi2P2 2 into 3 and 4 could be uncovered.
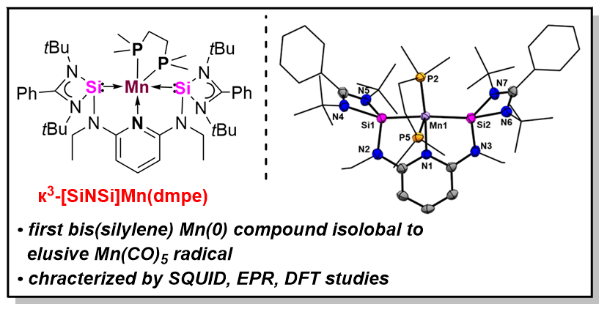
Using the potentially tridentate N,N’-bis(N-heterocyclic silylene)pyridine [SiNSi] pincer-type ligand, 2,6-N,N’-diethyl-bis[N,N’-di-tert-butyl(phenylamidinato)silylene] diaminopyridine, led to the first isolable bis(silylene)pyridine-stabilized manganese(0) complex, {к3-[SiNSi]Mn(dmpe)} 4 (dmpe = (Me2P)2C2H4), which represents an isolobal 17 VE analogue of the elusive Mn(CO)5 radical.
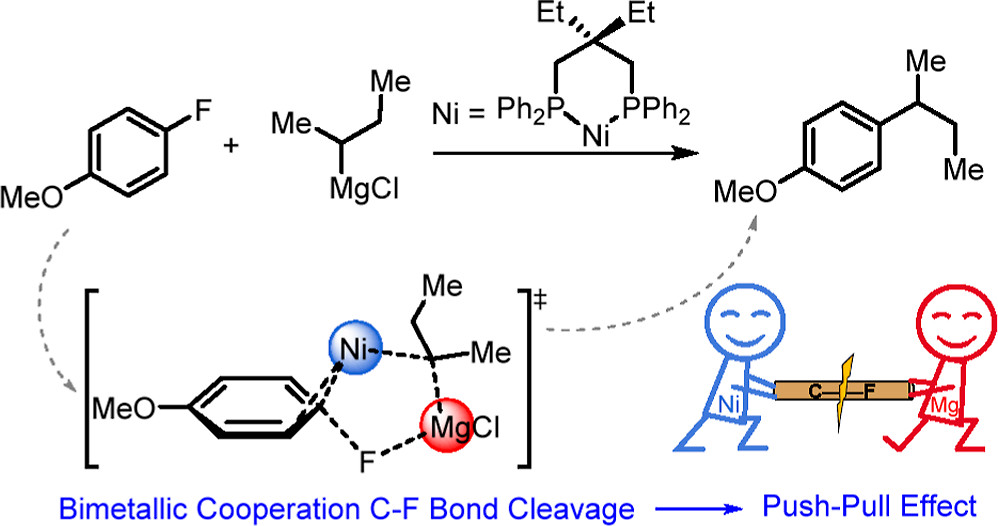
The Ni-catalyzed Kumada–Tamao–Corriu (KTC) cross-coupling between aryl fluorides and alkyl Grignard reagents has been used to achieve a highly selective Csp2–Csp3 bond construction via the carbon–fluorine (C–F) bond activation. However, the detailed mechanism of this groundbreaking KTC reaction remains unclear. Herein, we perform a series of analyses by density functional theory (DFT) calculations in order to understand the reaction mechanisms for the selective activation of a highly inert C–F bond by Ni catalysts with bidentate phosphorus ligands.
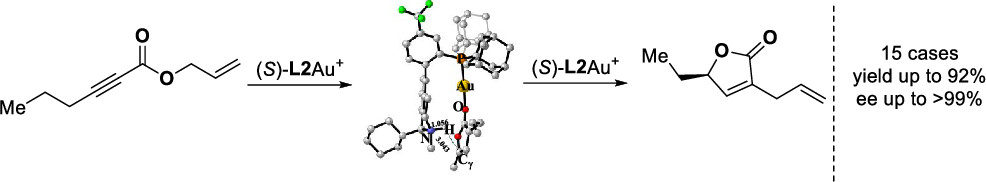
A highly efficient construction of chiral γ-substituted α-allyl-α,β-butenolides with up to >99% enantiomeric excess from readily available allylic ynoates is realized. In this asymmetric gold catalysis, the cationic gold(I) catalyst featuring a bifunctional phosphine ligand enables a four-step cascade which permits the conversion of a diverse array of allylic ynoates into valuable chiral α,γ-disubstituted α,β-butenolides.
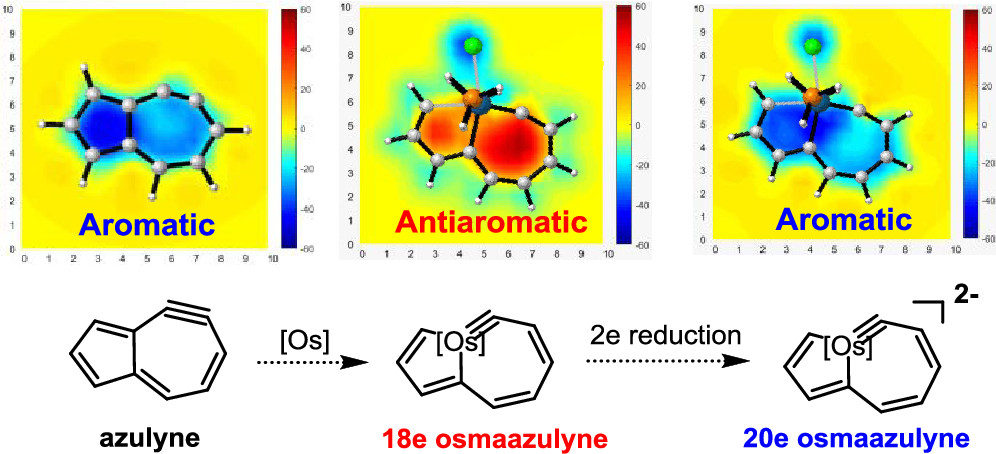
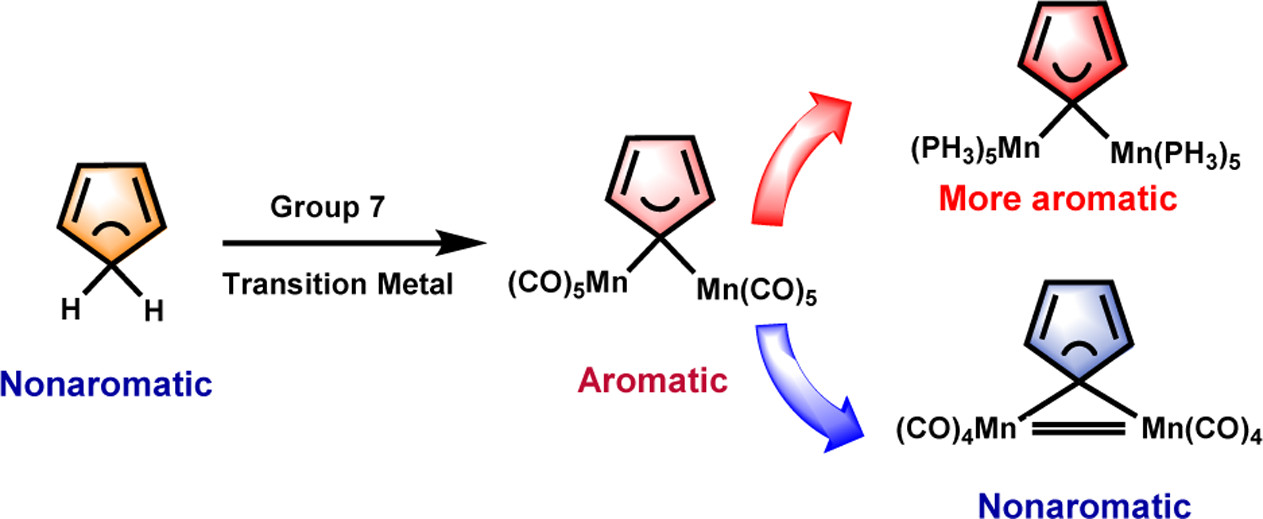
The 18-electron rule states that metal complexes with 18 valence electron metal centers are thermodynamically stable because nine valence orbitals of transition metals including one s orbital, three p orbitals, and five d orbitals can collectively accommodate 18 electrons, achieving the same electron configuration as the noble gas in the period. Thus, 20-electron compounds are extremely rare due to a violation of such a rule.
Activation of atmospherically abundant dinitrogen (N2) under mild conditions has been a great challenge in chemistry for decades because of the significantly strong N≡N triple bond. The traditional strategy of N2 activation was mainly limited to metallic species until the ground-breaking achievement of N2 activation by two-coordinated borylenes was achieved experimentally in 2018. On the other hand, carborane derivatives have attracted considerable interest for small-molecule activation. Still, the utilization of carborane derivatives in N2 activation remains elusive.
Copyright © 2025,
Theme Originally Created by Devsaran
