Probing the Hyperconjugative Aromaticity of Cyclopentadiene and Pyrroliums Containing Group 7 Transition Metal Substituents
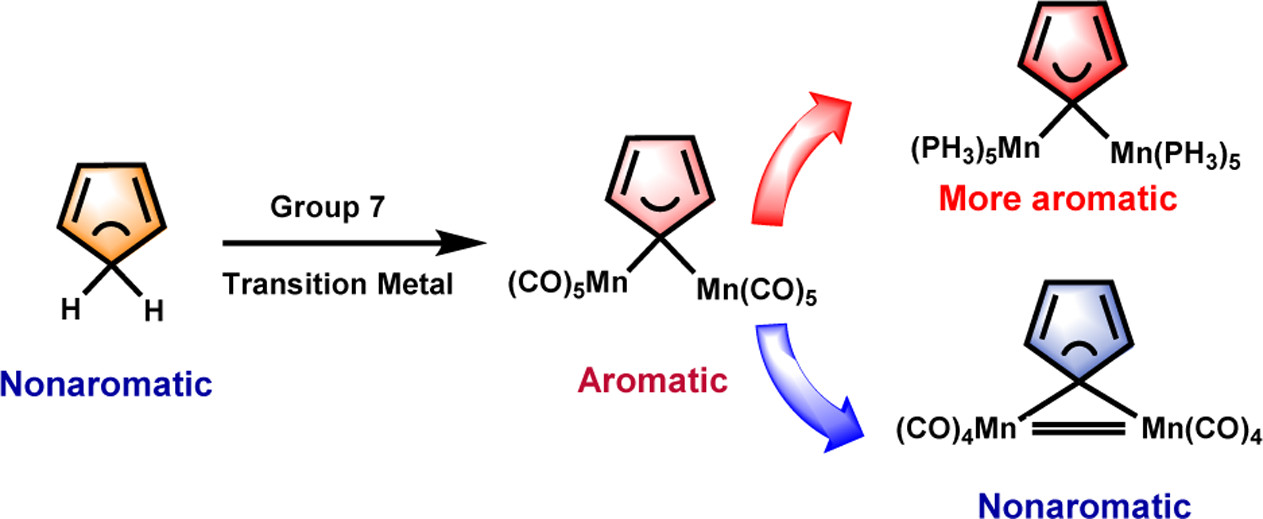
Aromaticity and hyperconjugation are two fundamental concepts in chemistry. Combining them together led to the proposal of the concept of hyperconjugative aromaticity by Mulliken in 1939. Now, it has been attracting considerable attention from both theoretical and experimental chemists. Recently, the concept of hyperconjugative aromaticity has been extended from main-group substituents to transition metal systems including groups 9, 10, and 11 transition metal substituents. Here, we report the hyperconjugative aromaticity in cyclopentadienes and pyrroliums containing group 7 transition metal substituents through density functional theory (DFT) calculations. It is found that the metal–metal bonding interaction can significantly reduce the aromaticity in cyclopentadienes, whereas the stronger σ-donor ligand, bridged hydride, and carbonyls can enhance aromaticity. All these findings expand the scope of the concept of hyperconjugative aromaticity, enriching aromatic organometallic chemistry.
