Nature Chemistry
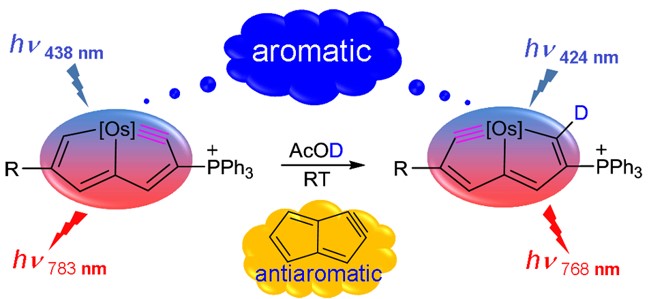


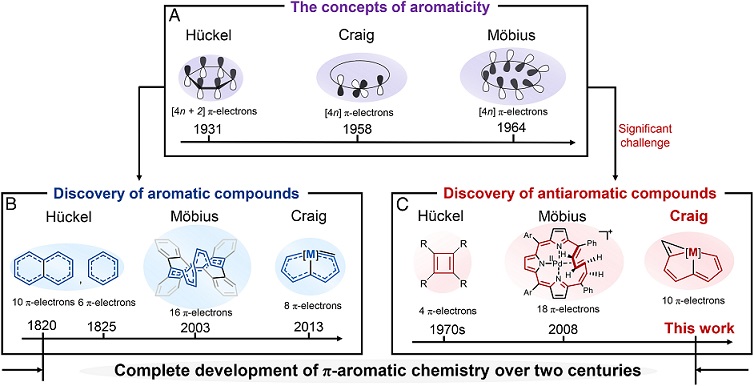
Antiaromaticity is extended from aromaticity as a complement to describe the unsaturated cyclic molecules with antiaromatic destabilization. To prepare antiaromatic species is a particularly challenging goal in synthetic chemistry because of the thermodynamic instability of such molecules. Among that, both Hückel and Möbius antiaromatic species have been reported, whereas the Craig one has not been realized to date. Here, we report the first example of planar Craig antiaromatic species.
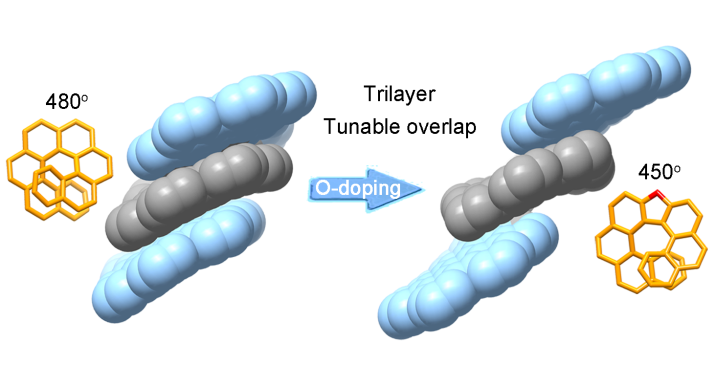
The synthesis of well-defined nanocarbon multilayers, beyond the bilayer structure, is still a challenging goal. Herein, two trilayer nanographenes were synthesized by covalently linking nanographene layers through helicene bridges. The structural characterization of the trilayer nanographenes revealed a compact trilayer-stacked architecture.
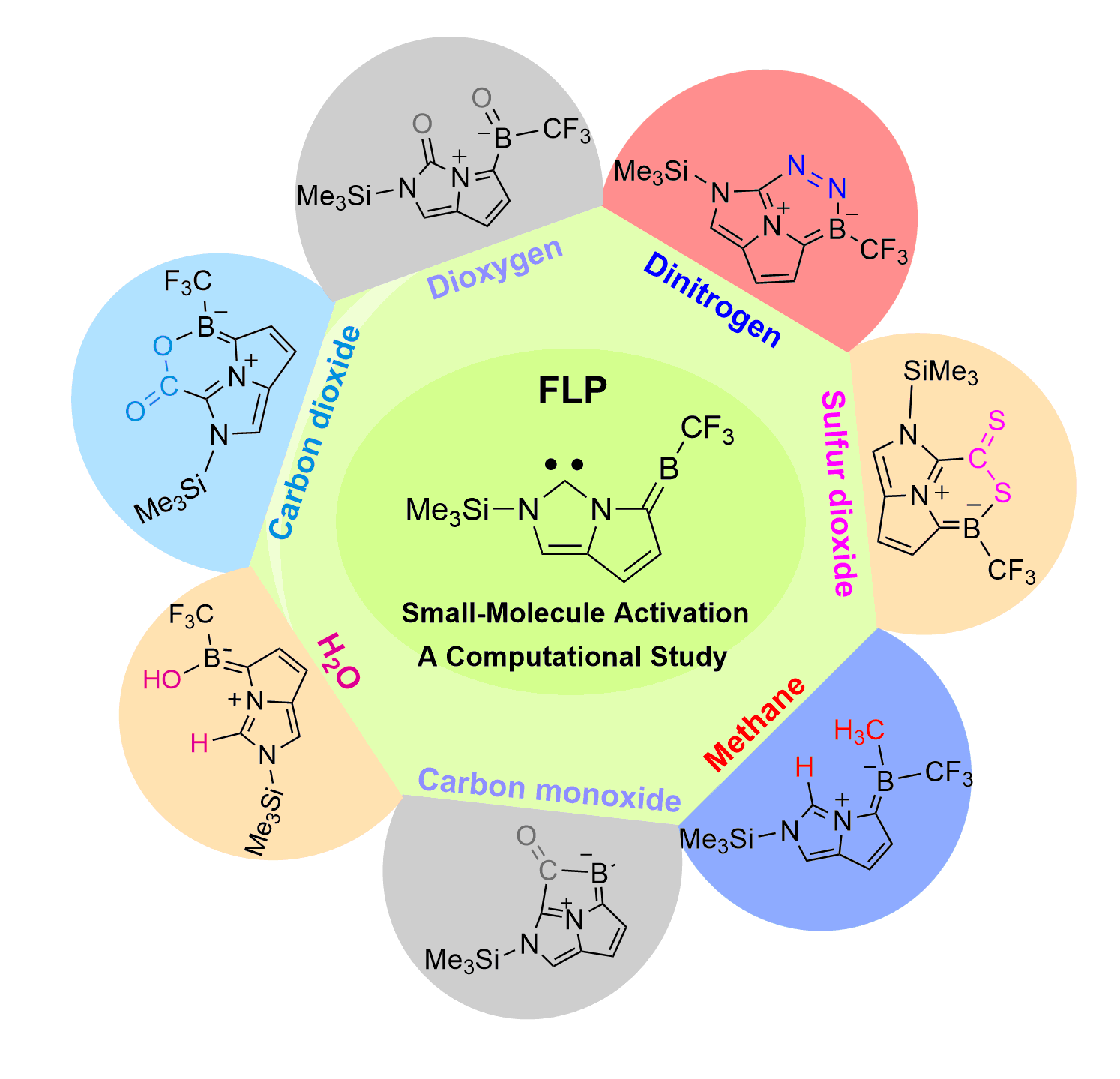
Dinitrogen (N2) activation is particularly challenging under ambient conditions because of its large highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) gap (10.8 eV) and high bond dissociation energy (945 kJ mol–1) of the NΞN triple bond, attracting considerable attention from both experimental and theoretical chemists. However, most effort has focused on metallic systems. In contrast, nitrogen activation by frustrated Lewis pairs (FLPs) has been initiated recently via theoretical calculations.
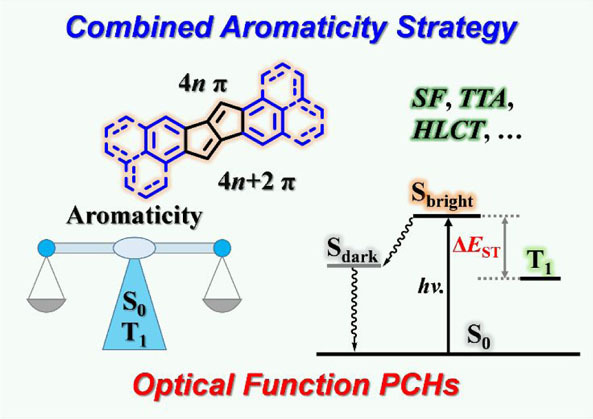
Understanding the structure–property relationships in polycyclic conjugated hydrocarbons (PCHs) is crucial for controlling their electronic properties and developing new optical function materials. Aromaticity is a fundamentally important and intriguing property of numerous organic chemical structures and has stimulated a myriad of experimental and theoretical investigations. Exploiting aromaticity rules for the rational design of optoelectronic materials with the desired photophysical characteristics is a challenging yet fascinating task.
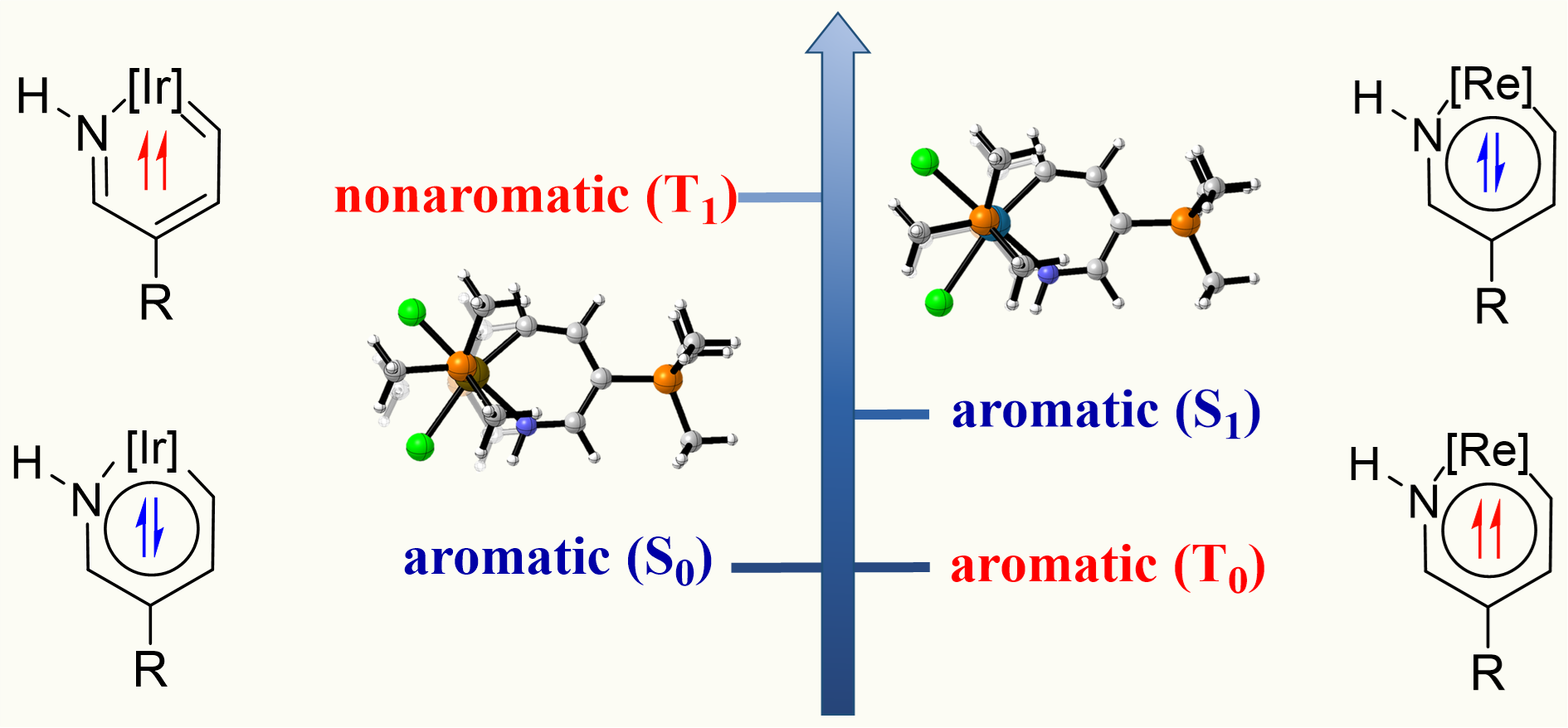
In general, compounds exhibit one-state aromaticity in either the ground or excited state according to the Hückel’s and Baird’s rules. Thus, species with two-state aromaticity in the lowest singlet and triplet states (termed as adaptive aromaticity) are rare. Understanding the underlying mechanism for achieving adaptive aromaticity is important to enrich this rare family. Here we carry out density functional theory (DFT) calculations to probe the origin of adaptive aromaticity in metallapyridiniums.
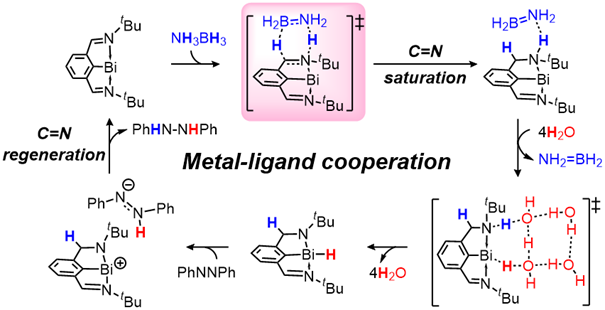
Transfer hydrogenation of azobenzene with ammonia borane mediated by pincer bismuth complex 1 were systematically investigated through density functional theory calculations. An unusual metal-ligand cooperation mechanism was disclosed, in which the saturation/regeneration of the C=N functional group on the pincer ligand plays an essential role. The reaction is initiated by the hydrogenation of the C=N bond (saturation) with ammonia borane to afford 3CN, which is the rate-determining step with Gibbs energy barrier (ΔG≠) and Gibbs reaction energy (ΔG) of 25.6 and -7.3 kcal/mol, respectively.
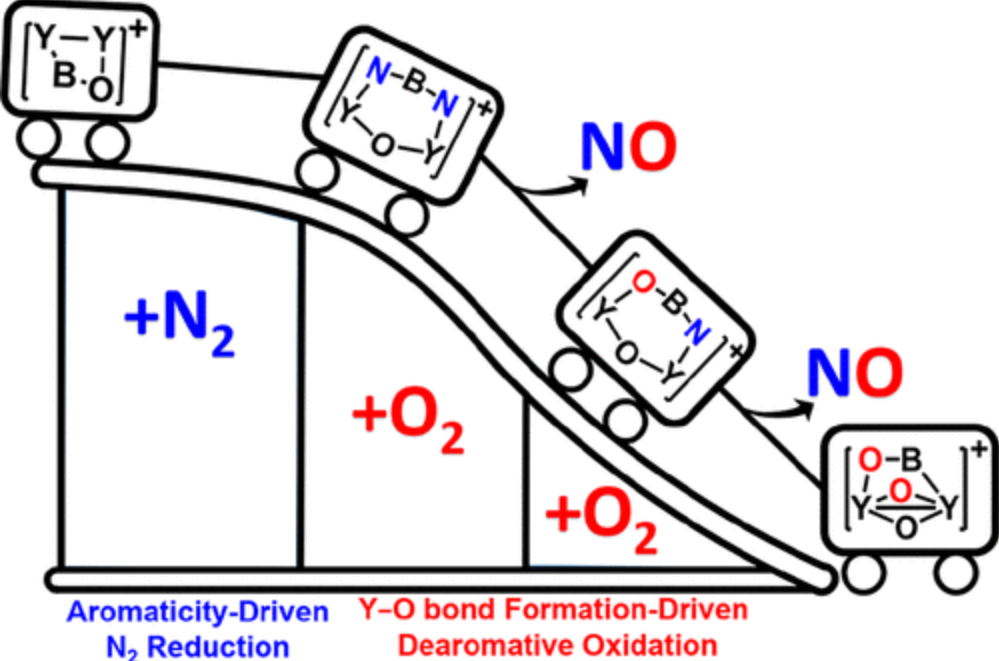
The conversion of dinitrogen to more useful and reactive molecules has been the focus of intense research by chemists. In contrast to reductive N2 fixation, direct oxidation of N2 by O2 to nitric oxide under mild conditions via a thermochemical process is extremely challenging. Herein, we report the first example of N2 and O2 activation and coupling under thermochemical conditions through the remarkable ability of Y2BO+ to react with one N2 and two O2 molecules.
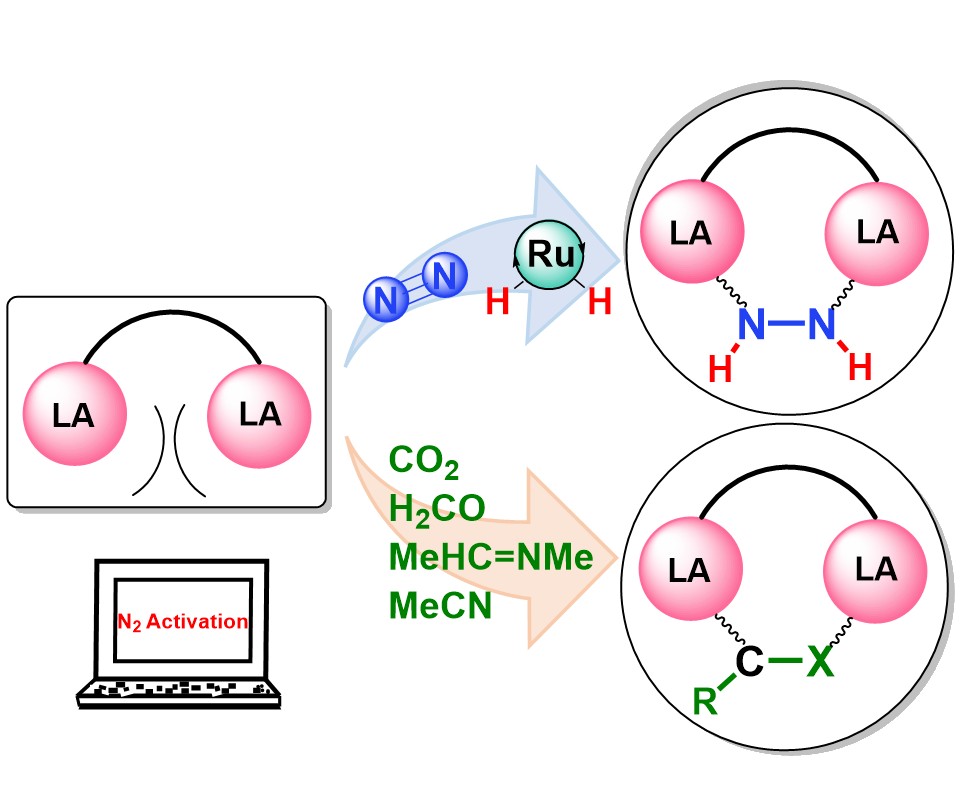
For decades, N2 activation and functionalization have required the use of transition metal complexes. Thus, it is one of the most challenging projects to activate the abundant dinitrogen through metal-free systems under mild conditions. Here, we demonstrate a proof-of-concept study on the catalytic hydrogenation of dinitrogen (with an activation energy as low as 15.3 kcal mol -1 ) initiated by a dual Lewis acid (DLA) via density functional theory (DFT) calculations.
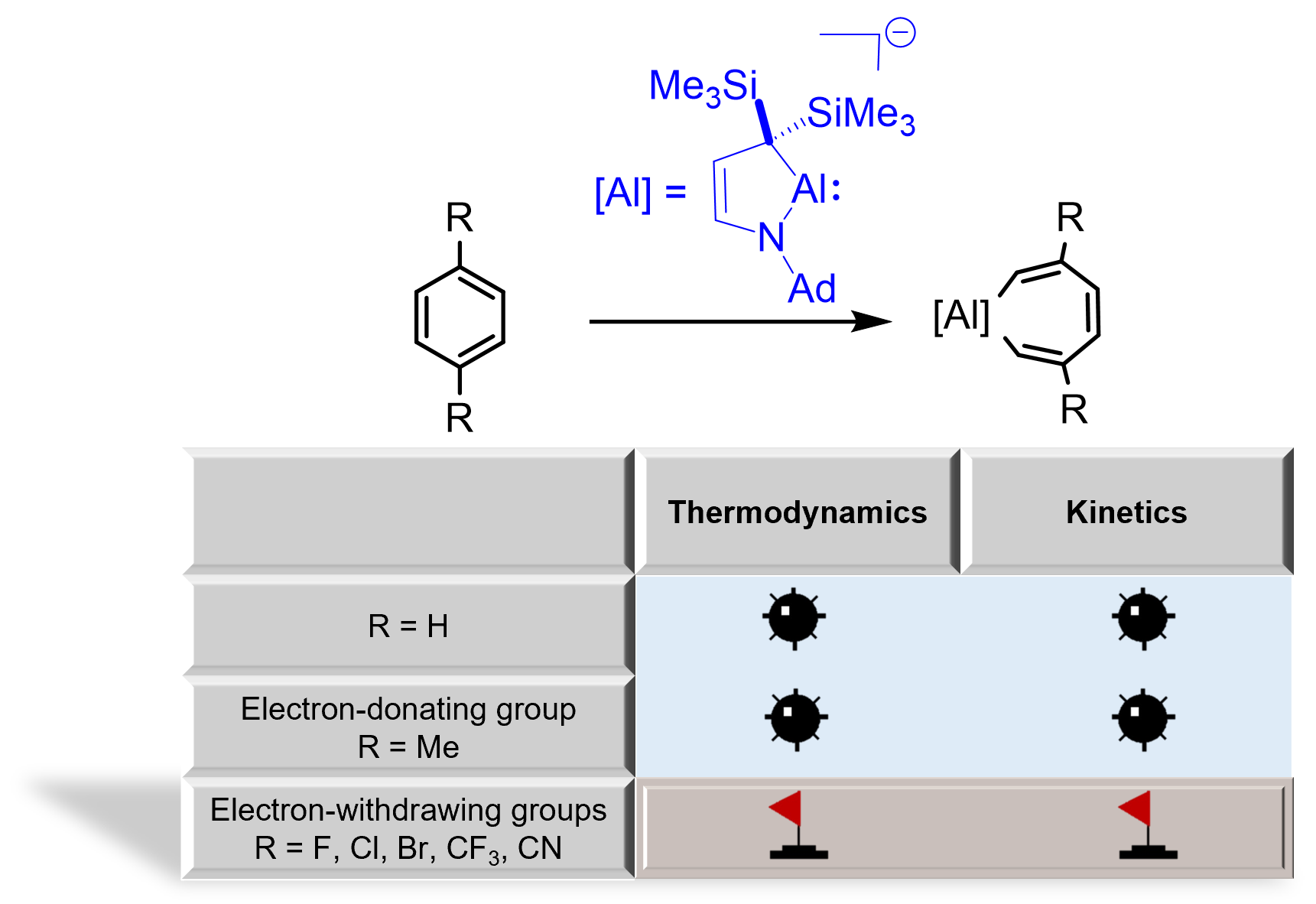
The oxidative addition of C-C bonds in aromatic hydrocarbons by low valent main group species has attracted considerable attention from both theoretical and experimental chemists due to the big challenge in breaking their aromaticity. Here, we demonstrate a general strategy to break the C-C bonds in benzene by cyclic (alkyl)(amino)aluminyl anion via density functional theory (DFT) calculations.
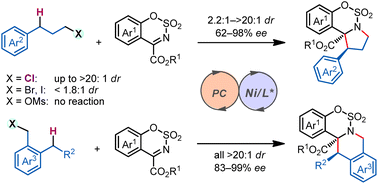
Asymmetric C(sp3)–H functionalization has emerged as a useful tool for simultaneous installation of functionality and chirality onto hydrocarbon units. Stereodiscrimination in reactions between a strong C(sp3)–H bond and a prochiral substrate, potentially forging vicinal stereogenic centers in a single step, however, remains a significant challenge. We report here a photocatalytic diastereo- and enantioselective C(sp3)–H functionalization/intramolecular cyclization reaction.
Copyright © 2025,
Theme Originally Created by Devsaran
