Singlet Fission in a Pyrrole-Fused Cross-Conjugated Skeleton with Adaptive Aromaticity
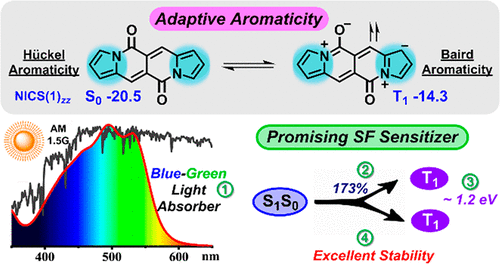
Singlet fission (SF) materials hold the potential to increase the power conversion efficiency of solar cells by reducing the thermalization of high-energy excited states. The major hurdle in realizing this potential is the limited scope of SF-active materials with high fission efficiency, suitable energy levels, and sufficient chemical stability. Herein, using theoretical calculation and time-resolved spectroscopy, we developed a highly stable SF material based on dipyrrolonaphthyridinedione (DPND), a pyrrole-fused cross-conjugated skeleton with a distinctive adaptive aromaticity (dual aromaticity) character. The embedded pyrrole ring with 4n+2 π-electron features aromaticity in the ground state, while the dipole resonance of the amide bonds promotes a 4n π-electron Baird’s aromaticity in the triplet state. Such an adaptive aromaticity renders the molecule efficient for the SF process [E(S1) ≥ 2E(T1)] without compromising its stability. Up to 173% triplet yield, strong blue-green light absorption, and suitable triplet energy of 1.2 eV, as well as excellent stability, make DPND a promising SF sensitizer toward practical applications.
