Achieving Adaptive Aromaticity in Cyclo[10]carbon by Screening Cyclo[n]carbon (n = 8‐24)
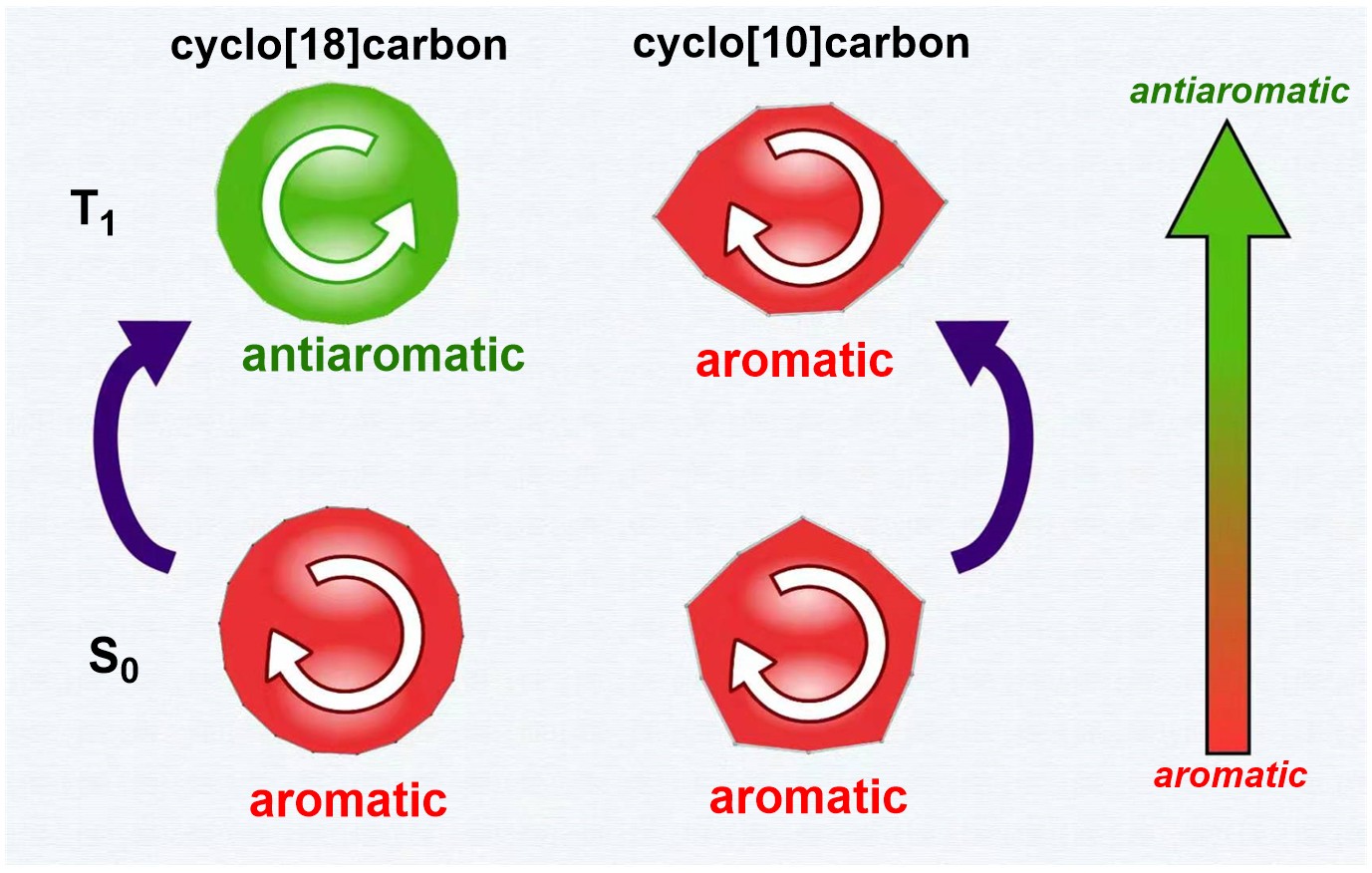
Discovery of species with adaptive aromaticity (being aromatic in both the lowest singlet and triplet states) is particularly challenging as cyclic species are generally aromatic either in the ground state or in the excited state only according to Hückel’s and Baird’s rules. Inspired by recent realization of cyclo[18]carbon, here we demonstrate that cyclo[10]carbon possesses adaptive aromaticity by screening cyclo[n]carbon (n = 8‐24), which is supported by nucleus independent chemical shift (NICS), anisotropy of the induced current density (ACID), electron localization function (ELF π ) and electron density of delocalized bonds (EDDB) analyses. Further study reveals that the lowest triplet state of cyclo[10]carbon is formed by in‐plane ππ* excitation. Thus, the major contribution to the aromaticity from out‐of‐plane π molecular orbitals does not change significantly in the lowest singlet state. Our findings highlight a crucial role of out‐of‐plane π orbitals in maintaining aromaticity for both the lowest singlet and triplet states as well as the aromaticity dependence on the number of the carbon in cyclo[n]carbon.
