Nature Chemistry
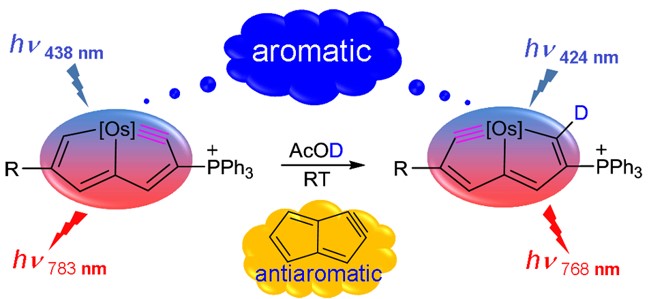


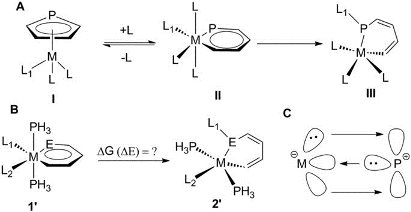
Metallabenzenes have attracted considerable interest of both theoretical and experimental chemists. However, metallaphosphabenzene has never been synthesized. Thus, understanding the origin of the challenge of synthesizing metallaphosphabenzene is particularly urgent for experimentalists. Now density functional theory (DFT) calculations have been carried out to examine this issue.
Polymetalated aromatic compounds are particularly challenging synthetic goals because of the limited thermodynamic stability of polyanionic species arising from strong electrostatic repulsion between adjacent carbanionic sites. Here we describe a facile synthesis of two polyaurated complexes including a tetra-aurated indole and an octa-aurated benzodipyrrole. The imido trinuclear gold(I) moiety exhibits nucleophilicity and undergoes an intramolecular attack on a gold(I)-activated ethynyl to generate polyanionic heteroaryl species.
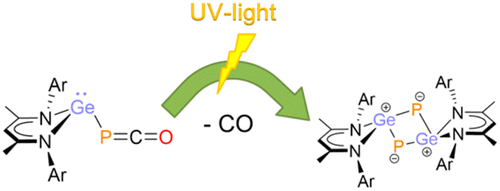
The heavier cyclobutadiene analogue 2,4-digerma-1,3-diphosphacyclobutadiene ([L12Ge2P2], 4; L1 = CH{(CMe)(2,6-iPr2C6H3N)}2), featuring a planar Ge2P2 four-membered ring, has been synthesized via the elimination of carbon monoxide from the corresponding phosphaketenyl germylene [L1GePCO] (2) under UV irradiation.
DOI: 10.1039/C6QO90012G

http://pubs.rsc.org/en/content/articlelanding/2016/qo/c6qo90012g#!divAbstract
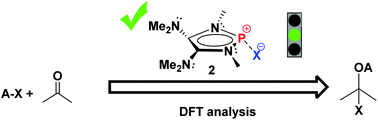
Density functional theory (DFT) calculations were carried out to investigate the hydridic character of several main group hydrides. A P-hydrido-1,3,2-diazaphospholene 1f with two π-electron donor amino groups on the heterocyclic skeleton framework performs as a strong hydride donor owing to the significant n(N)–σ*(P–H) hyperconjugation. The natural bond orbital analysis reveals that high π-electron delocalization exists in both 1f and the corresponding stable phosphenium Ef+.
As one of the most important chemical reactions, SN2 reactions play a central role in both synthetic chemistry and the development of mechanistic paradigms. Most of the previous attention has centered on substitution at carbon, whereas displacement at nitrogen is clearly less studied and less understood. The SN2@N reactions have enormous synthetic potential, especially in heterocyclic chemistry, which is a cornerstone of medicinal chemistry, and has been receiving increasing attention, both experimentally and computationally.
For details, please check the link at http://pubs.rsc.org/en/content/articlelanding/2016/cc/c6cc90010k#!divAbstract
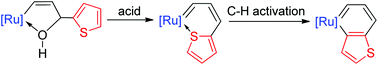
The first ruthenabenzothiophenes have been achieved via the C–H activation of thiophene. These species feature high thermal stability and resistance of a moderate oxidant, which constitute valuable addition to the rare metallaaromatic containing second-row transition metals.
http://pubs.rsc.org/en/content/articlelanding/2015/dt/c5dt04557f#!divAbstract
For details, please check the link at http://pubs.rsc.org/en/content/articlelanding/2015/dt/c5dt04557f#!divAbstract
Copyright © 2025,
Theme Originally Created by Devsaran
