Mechanism of Nickel-Catalyzed Selective C–N Bond Activation in Suzuki-Miyaura Cross-Coupling of Amides: A Theoretical Investigation
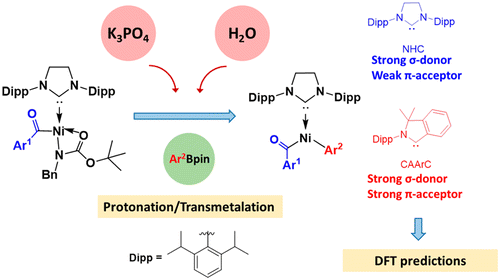
In textbooks, the low reactivity of amides is attributed to the strong resonance stability. However, Garg and co-workers recently reported the Ni-catalyzed activation of robust amide C–N bonds, leading to conversions of amides into esters, ketones, and other amides with high selectivity. Among them, the Ni-catalyzed Suzuki-Miyaura coupling (SMC) of N-benzyl-N-tert-butoxycarbonyl (N-Bn-N-Boc) amides with pinacolatoboronate (PhBpin) was performed in the presence of K3PO4 and water. Water significantly enhanced the reaction. With the aid of density functional theory (DFT) calculations, the present study explored the mechanism of the aforementioned SMC reaction as well as analyzed the weakening of amide C–N bond by N-functionalization. The most favorable pathway includes four basic steps: oxidative addition, protonation, transmetalation, and reductive elimination. Comparing the base- and water-free process, the transmetalation step with the help of K3PO4 and water is significantly more facile. Water efficiently protonates the basic N(Boc) (Bn) group to form a neutral HN(Boc) (Bn), which is easily removed. The transmetalation step is the rate-determining step with an energy barrier of 25.6 kcal/mol. Further, a DFT prediction was carried out to investigate the full catalytic cycle of a cyclic (amino) (aryl)carbene in the Ni-catalyzed SMC of amides, which provided clues for further design of catalysts.
