Mechanism of Nickel-Catalyzed Selective C–N Bond Activation in Suzuki-Miyaura Cross-Coupling of Amides: A Theoretical Investigation
Submitted by Jun Zhu on Thu, 11/03/2016 - 15:05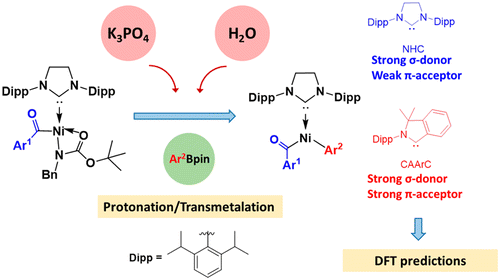
In textbooks, the low reactivity of amides is attributed to the strong resonance stability. However, Garg and co-workers recently reported the Ni-catalyzed activation of robust amide C–N bonds, leading to conversions of amides into esters, ketones, and other amides with high selectivity. Among them, the Ni-catalyzed Suzuki-Miyaura coupling (SMC) of N-benzyl-N-tert-butoxycarbonyl (N-Bn-N-Boc) amides with pinacolatoboronate (PhBpin) was performed in the presence of K3PO4 and water. Water significantly enhanced the reaction.