σ-Aromaticity in an Unsaturated Ring: Osmapentalene Derivatives Containing a Metallacyclopropene Unit
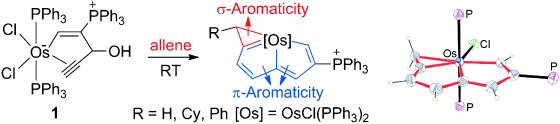
In general, aromaticity can be clarified as π- and σ-aromaticity according to the type of electrons with major contributions. The traditional π-aromaticity generally describes the π-conjugation in fully unsaturated rings whereas σ-aromaticity may stabilize fully saturated rings with delocalization caused by σ-electron conjugation. Reported herein is an example of σ-aromaticity in an unsaturated three-membered ring (3 MR), which is supported by experimental observations and theoretical calculations. Specifically, when the 3 MR in cyclopropaosmapentalene is cleaved by ethane through two isodesmic reactions, both of them are highly endothermic (+29.7 and +35.0 kcal mol−1). These positive values are in sharp contrast to the expected exothermicity, thus indicating aromaticity in the 3 MR. Further nucleus-independent chemical shift and anisotropy of the current-induced density calculations reveal the nature of σ-aromaticity in the unsaturated 3 MR.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201411220/abstract
