Nature Chemistry
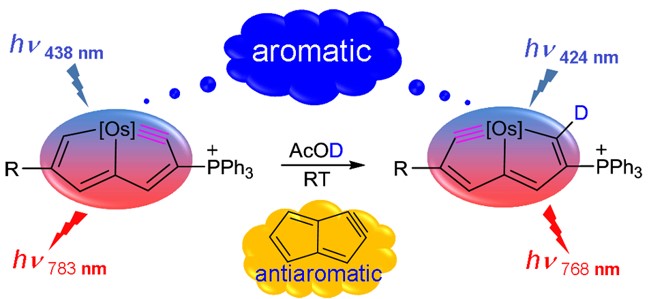



Aromaticity, a highly stabilizing feature of molecules with delocalized electrons in closed circuits, is generally restricted to ‘Hückel’ systems with 4n+2 mobile electrons. Although the Möbius concept extends the principle of aromaticity to 4n mobile electron species, the rare known examples have complex, twisted topologies whose extension is unlikely. Here we report the realization of osmapentalenes, the first planar Möbius aromatic complexes with 16 and 18 valence electron transition metals.
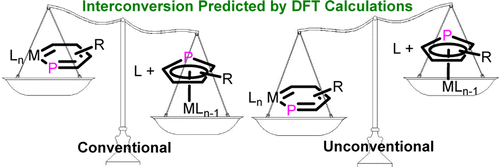
Metallaaromatics have attracted continuing interest of both theoretical and experimental chemists since the first metallabenzene was predicted by Hoffmann and isolated by Roper. In sharp contrast to metallabenzenes, metallaphosphabenzene (MPB) is much less developed and has not been synthesized so far. Thus, developing synthetic approaches is urgent. Here we present thorough density functional theory (DFT) calculations on the thermodynamics and kinetics of the rearrangement between MPBs and the corresponding η5-phosphacyclopentadiene (η5-PCp) complexes.
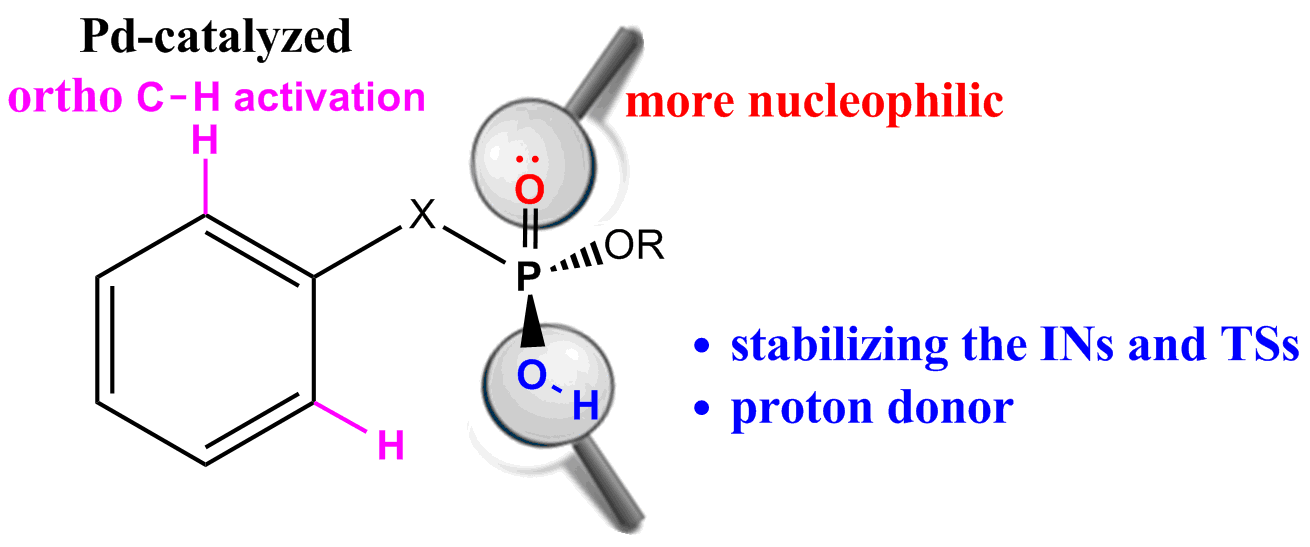
Density functional theory (DFT) calculations have been carried out on Pd-catalyzed phosphoryl-directed ortho-olefination to probe the origin of the significant reactivity difference between methyl hydrogen benzylphosphonates and dialkyl benzylphosphonates. The overall catalytic cycle is found to include four basic steps: C−H bond activation, transmetalation, reductive elimination and recycling of catalyst, each of which is constituted from different steps.
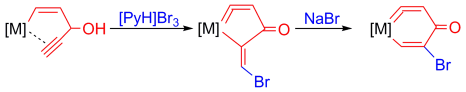
Highly stable five-membered metallacycloallenes were synthesized under mild conditions. Calculations revealed that the incorporation of transition-metal moieties relieves considerable strain and indicates a trend toward ring enlargement in the five-membered metallacycloallenes. Conversion into six-membered metallacycloallenes was confirmed experimentally.
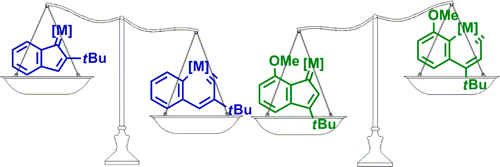
Metallaaromatics have attracted considerable interest of both theoretical and experimental chemists. However, there have been only two metallanaphthalynes isolated so far. Thus, developing new synthetic approaches is urgent. Here we present thorough density functional theory (DFT) calculations on the thermodynamics and kinetics of the isomerization between metallanaphthalynes and metal indenylidene complexes. The effects of metal centers, ligands, and substituents on the metallabicycles were examined systematically.
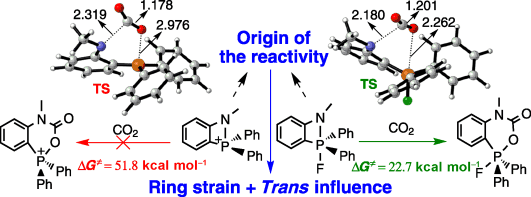
CO2 capture has attracted increasing attention owing to its contribution to global warming and climate change as a greenhouse gas. As an alternative strategy to transition-metal-based chemistry and catalysis, frustrated Lewis pairs have been developed to sequester CO2 efficiently under mild conditions. However, the mechanism of CO2 sequestration with amidophosphoranes remains unclear. Herein, we present a thorough density functional theory study on a series of amidophosphoranes.
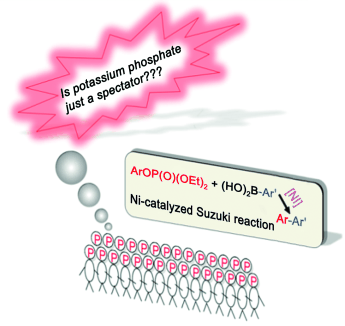
Spectator or actor? Density functional theory calculations were performed to examine the role of the base in the nickel-catalyzed cross-coupling of aryl phosphates with arylboronic acids. Potassium phosphate was found to not act as a spectator base but was involved in the transmetalation step, as shown by a lower barrier than that of a base-free process, owing to the activation of the carbonboron bond by the base. Further experimental observations support the theoretical findings.
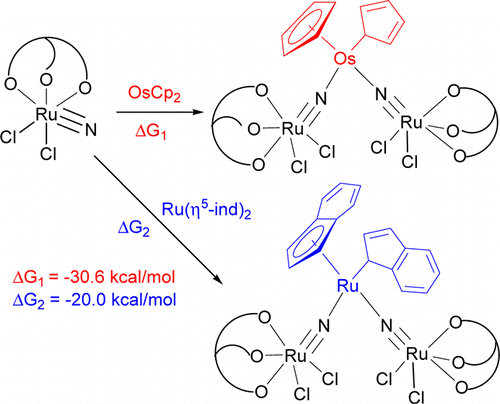
η5–η1 ring slippage of [OsCp2] (Cp = η5-C5H5) and [Ru(η5-ind)2] (ind = indenyl) resulting from reaction with the ruthenium(VI) nitride [Ru(LOEt)(N)Cl2] (1; LOEt– = [CoCp{P(O)(OEt)2}3]−) is reported. The treatment of [OsCp2] or [Ru(η5-ind)2] with 1 resulted in η5-η1 ring slippage of the cycloolefin ligands and formation of the trinuclear nitrido complexes [Cp(η1-C5H5)Os(NRuLOEtCl2)2] (2) or [(η5-ind)(η1-ind)Ru(NRuLOEtCl2)2] (3).
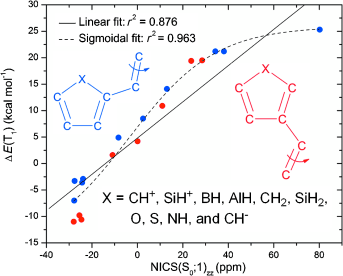
A density functional theory study on olefins with five-membered monocyclic 4n and 4n+2 π-electron substituents (C4H3X; X=CH+, SiH+, BH, AlH, CH2, SiH2, O, S, NH, and CH−) was performed to assess the connection between the degree of substituent (anti)aromaticity and the profile of the lowest triplet-state (T1) potential-energy surface (PES) for twisting about olefinic CC bonds. It exploited both Hückel’s rule on aromaticity in the closed-shell singlet ground state (S0) and Baird’s rule on aromaticity in the lowest ππ* excited triplet state.
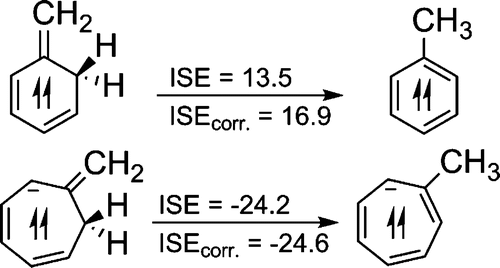
The many manifestations of aromaticity have long fascinated both experimentalists and theoreticians. Due to their degenerate half-filled MOs, triplet [n]annulenes with 4n π-electrons are also aromatic, but the degree of their stabilization has been difficult to quantify. The isomerization stabilization energy (ISE) method has been applied to evaluate the triplet aromaticity. The reliability of this approach is indicated by the strong correlation of the ISE results with NICS(1)zz, a magnetic indicator of triplet state aromaticity.
Copyright © 2025,
Theme Originally Created by Devsaran
