Facile η5–η1 Ring Slippage of the Cycloolefin Ligands in Osmocene and Bis(η5-indenyl)ruthenium(II)
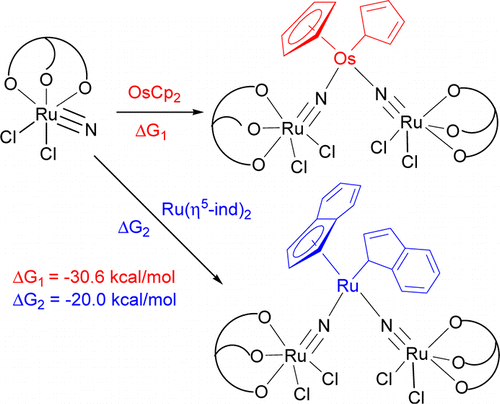
η5–η1 ring slippage of [OsCp2] (Cp = η5-C5H5) and [Ru(η5-ind)2] (ind = indenyl) resulting from reaction with the ruthenium(VI) nitride [Ru(LOEt)(N)Cl2] (1; LOEt– = [CoCp{P(O)(OEt)2}3]−) is reported. The treatment of [OsCp2] or [Ru(η5-ind)2] with 1 resulted in η5-η1 ring slippage of the cycloolefin ligands and formation of the trinuclear nitrido complexes [Cp(η1-C5H5)Os(NRuLOEtCl2)2] (2) or [(η5-ind)(η1-ind)Ru(NRuLOEtCl2)2] (3). No reactions were found between [OsCp2] and amines, such as pyridine and 2,2′-bipyridyl, or other metal nitrides, such as [Os(LOEt)(N)Cl2], indicating that the electrophilic property of 1 is essential for ring slippage. The crystal structures of 2 and 3 have been determined. The short Os–N distances in 2 [1.833(5) and 1.817(5) Å] and the (ind)Ru–N distances in 3 [1.827(5) and 1.852(5) Å] are indicative of multiple bond character, consistent with density functional theory (DFT) calculations. Therefore, 2 and 3 may be described by two resonance forms: RuVI–MII–RuVI and RuIV–MVI–RuIV (M = Os, Ru). Also, DFT calculations indicate that for the reaction of 1 with [OsCp2] or [Ru(η5-ind)2], η5–η1 ring slippage is energetically more favorable than the η5–η3 counterpart. The driving force for η5–η1 ring slippage is believed to be the formation of the strong M–N (M = Os, Ru) (multiple) bonds. By contrast, the same reaction with acetonitrile is energetically uphill, and thus no ring slippage occurs.
