Why Does Activation of the Weaker C═S Bond in CS2 by P/N-Based Frustrated Lewis Pairs Require More Energy Than That of the C═O Bond in CO2? A DFT Study
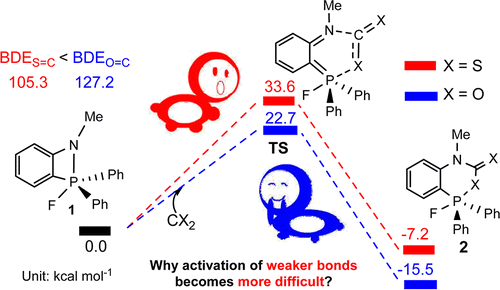
The sequestration of carbon disulfide (CS2), a common pollutant in environmental systems, is of great importance due to its physical harm to human beings. Compared with CO2 capture, that of CS2 is much less developed. The use of P/N-based frustrated Lewis pairs (FLPs) has been proven, both experimentally and theoretically, to be an alternative strategy to efficiently sequestrate CO2. Therefore, we pose the question of whether the analogue CS2 could also be sequestrated by the same FLPs, given that the C═S bond in CS2 is weaker than the C═O bond in CO2. Herein, we carry out a thorough DFT study to theoretically examine this hypothesis for a series of P/N-based FLPs. Our results reveal unexpectedly higher reaction barriers in CS2 capture by most of the P/N-based FLPs, although the bond dissociation energy of the C═S bond in CS2 (105.3 kcal mol–1) is smaller than that of the C═O bond in CO2 (127.2 kcal mol–1). The unexpected higher energy required for CS2 activation can be rationalized by its larger bond distortion and its reverse bond polarization, as revealed by energy decomposition analysis and natural bond orbital analysis, respectively. Our findings could be helpful for experimentalists investigating the sequestration of CS2 with P/N-based FLPs.
