Facile Dinitrogen and Dioxygen Cleavage by a Uranium(III) Complex: Cooperativity Between the Non‐innocent Ligand and the Uranium Center
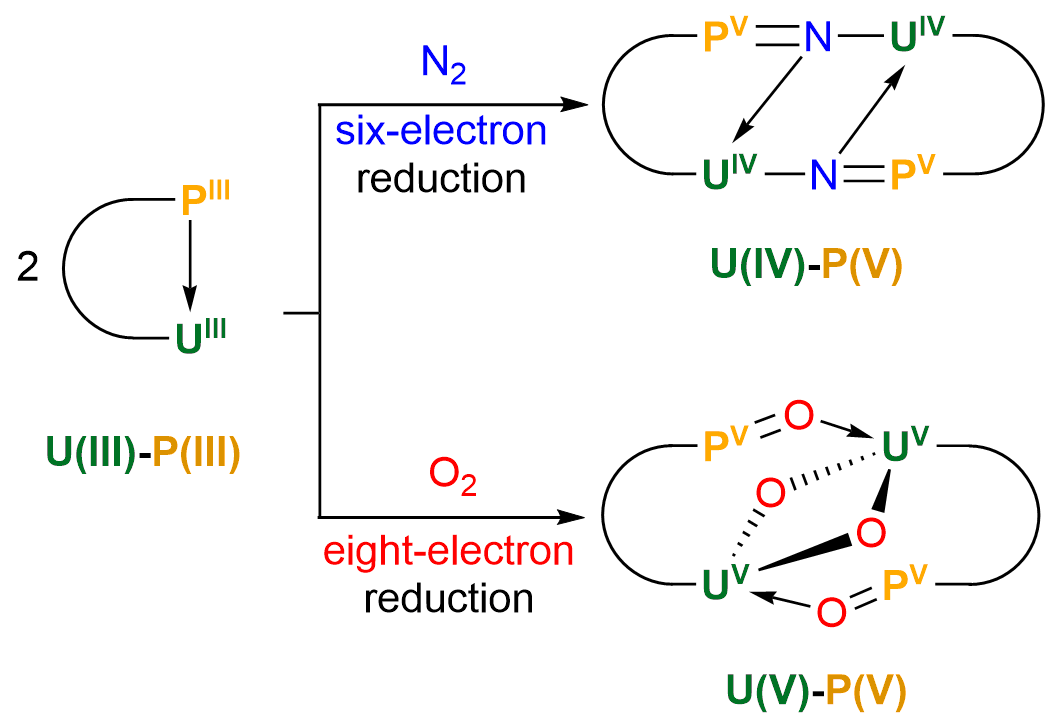
Activation of dinitrogen (N2, 78%) and dioxygen (O2, 21%) has fascinated chemists and biochemists for decades. The industrial conversion of N2 to ammonia requires extremely high temperatures and pressure. Here we report the first example of N2 and O2 cleavage by a uranium complex, [N(CH2CH2NPiPr2)3U]2(TMEDA), under ambient conditions without an external reducing agent. The N2 triple bond breaking implies a U(III)-P(III) six-electron reduction. The hydrolysis of the N2 reduction product allows the formation of ammonia or nitrogen-containing organic compound. The interaction between U(III) and P(III) in this molecule allows an eight-electron reduction of two O2 molecules. This study establishes that the combination of uranium and a low-valent nonmetal is a promising strategy to achieve a full N2 and O2 cleavage under ambient conditions, which may aid the design of new system for small molecules activation.
