Facile Dinitrogen and Dioxygen Cleavage by a Uranium(III) Complex: Cooperativity Between the Non‐innocent Ligand and the Uranium Center
Submitted by Jun Zhu on Wed, 09/16/2020 - 15:48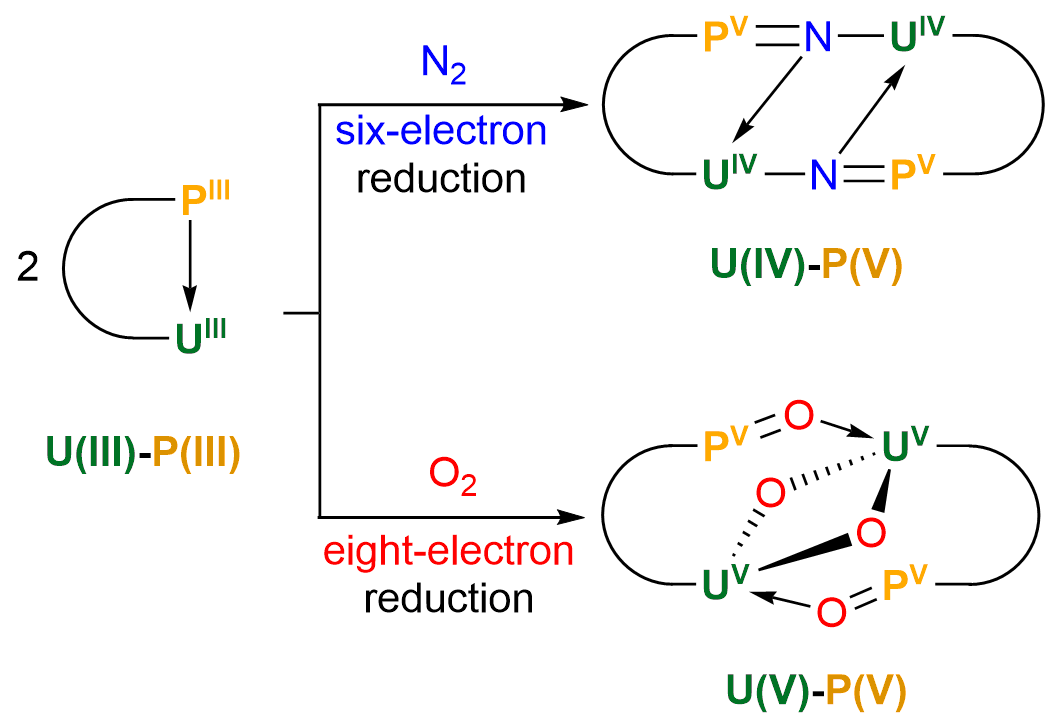
Activation of dinitrogen (N2, 78%) and dioxygen (O2, 21%) has fascinated chemists and biochemists for decades. The industrial conversion of N2 to ammonia requires extremely high temperatures and pressure. Here we report the first example of N2 and O2 cleavage by a uranium complex, [N(CH2CH2NPiPr2)3U]2(TMEDA), under ambient conditions without an external reducing agent. The N2 triple bond breaking implies a U(III)-P(III) six-electron reduction. The hydrolysis of the N2 reduction product allows the formation of ammonia or nitrogen-containing organic compound.
