Predicting Dinitrogen Activation by Boron Radical Cations
Submitted by Jun Zhu on Tue, 02/04/2025 - 09:36
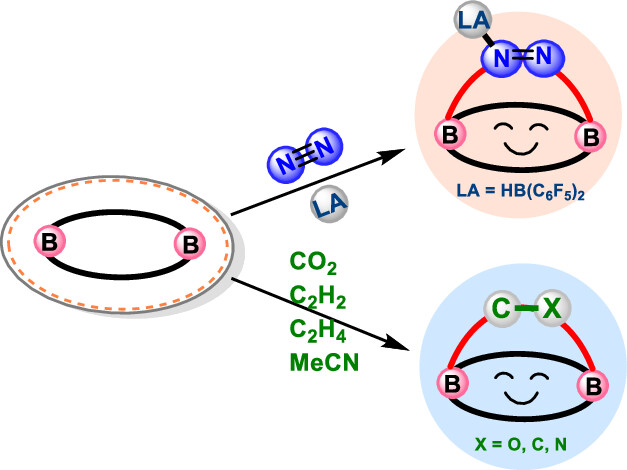
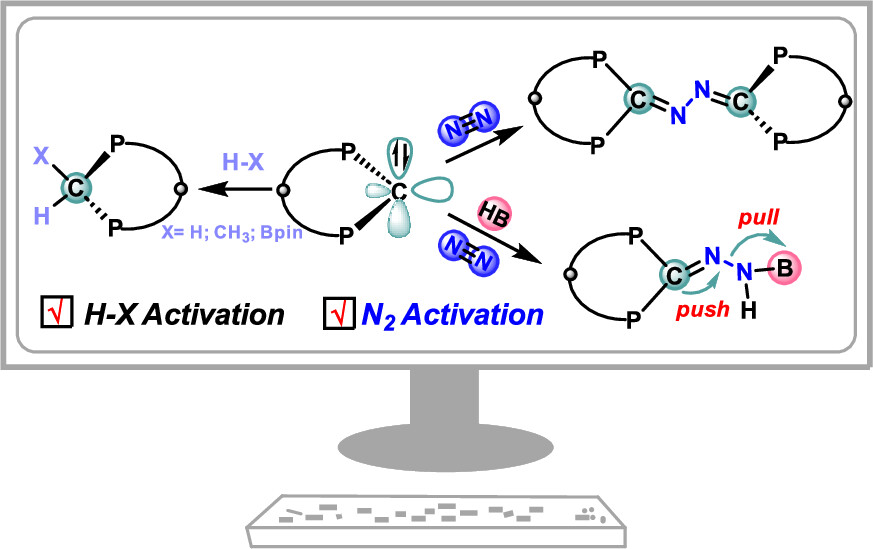
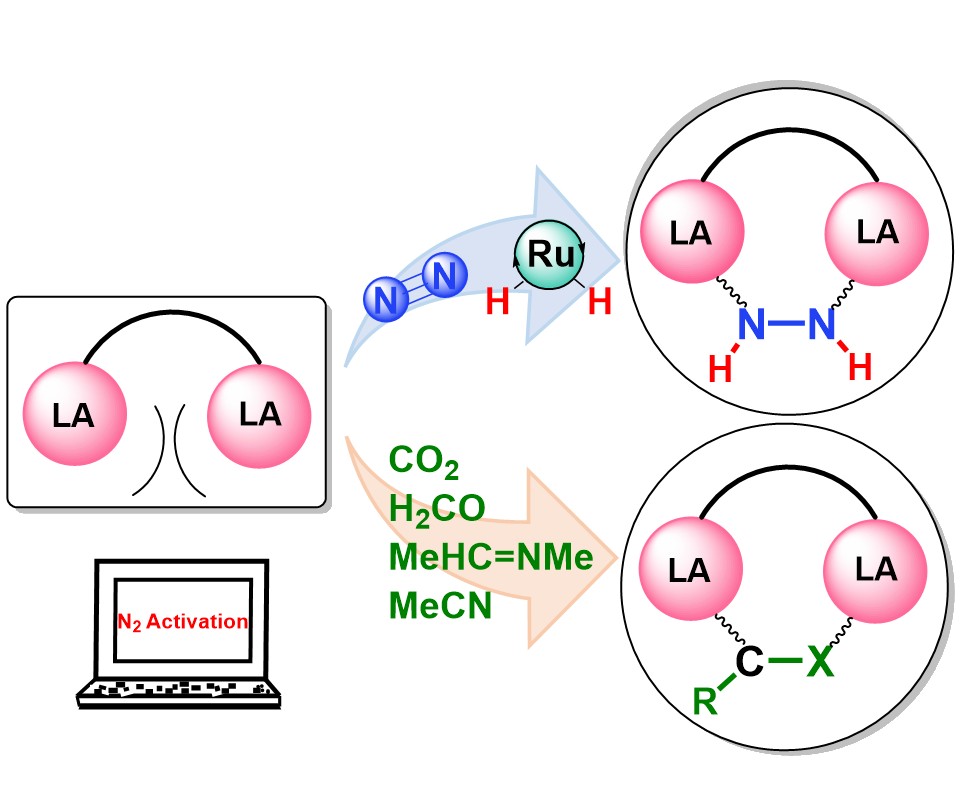
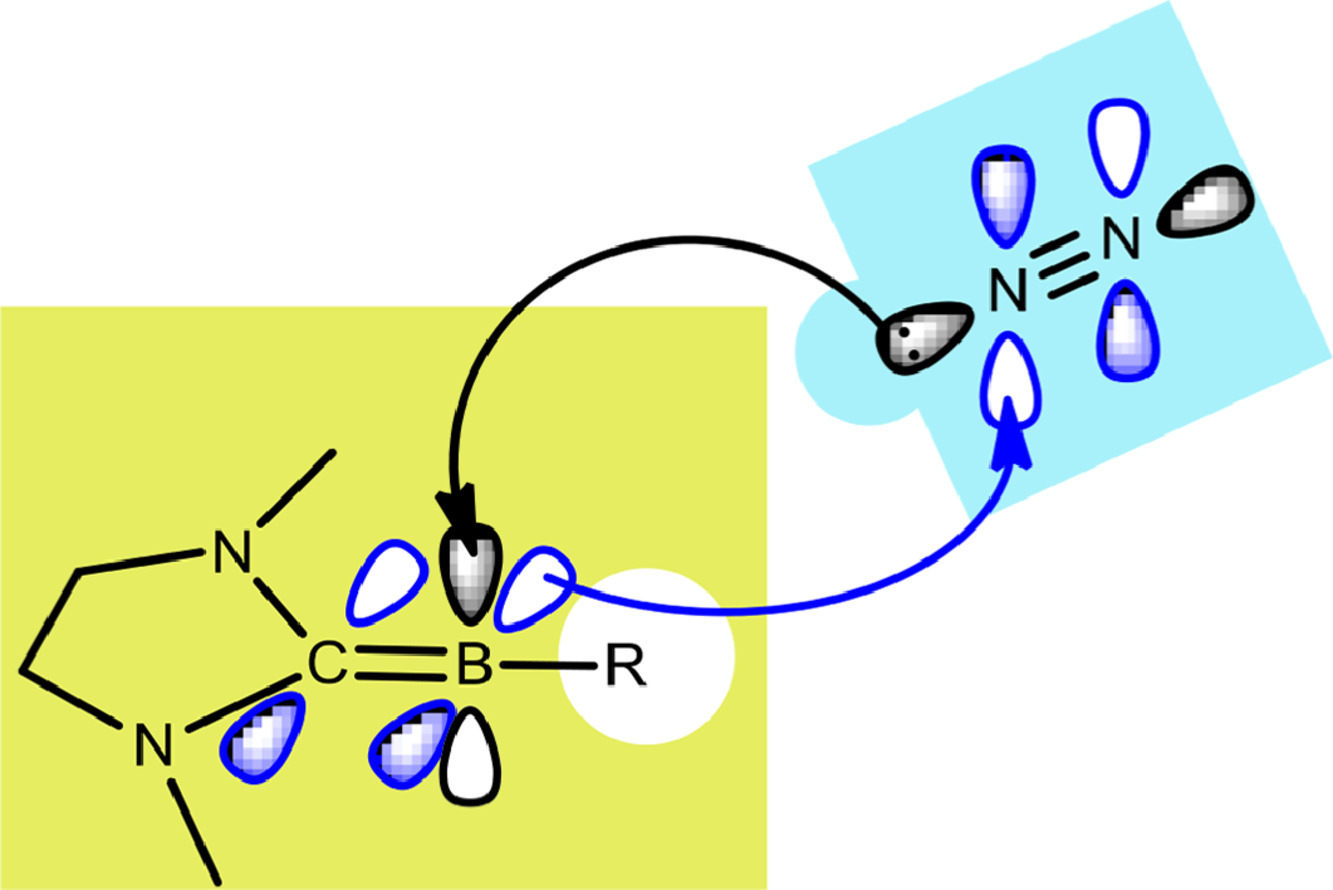
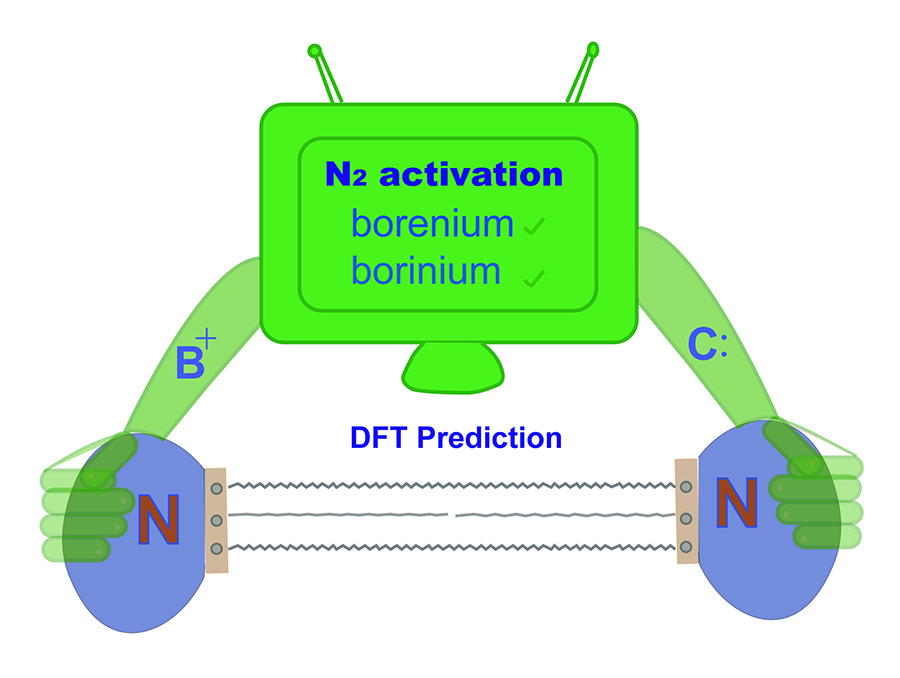
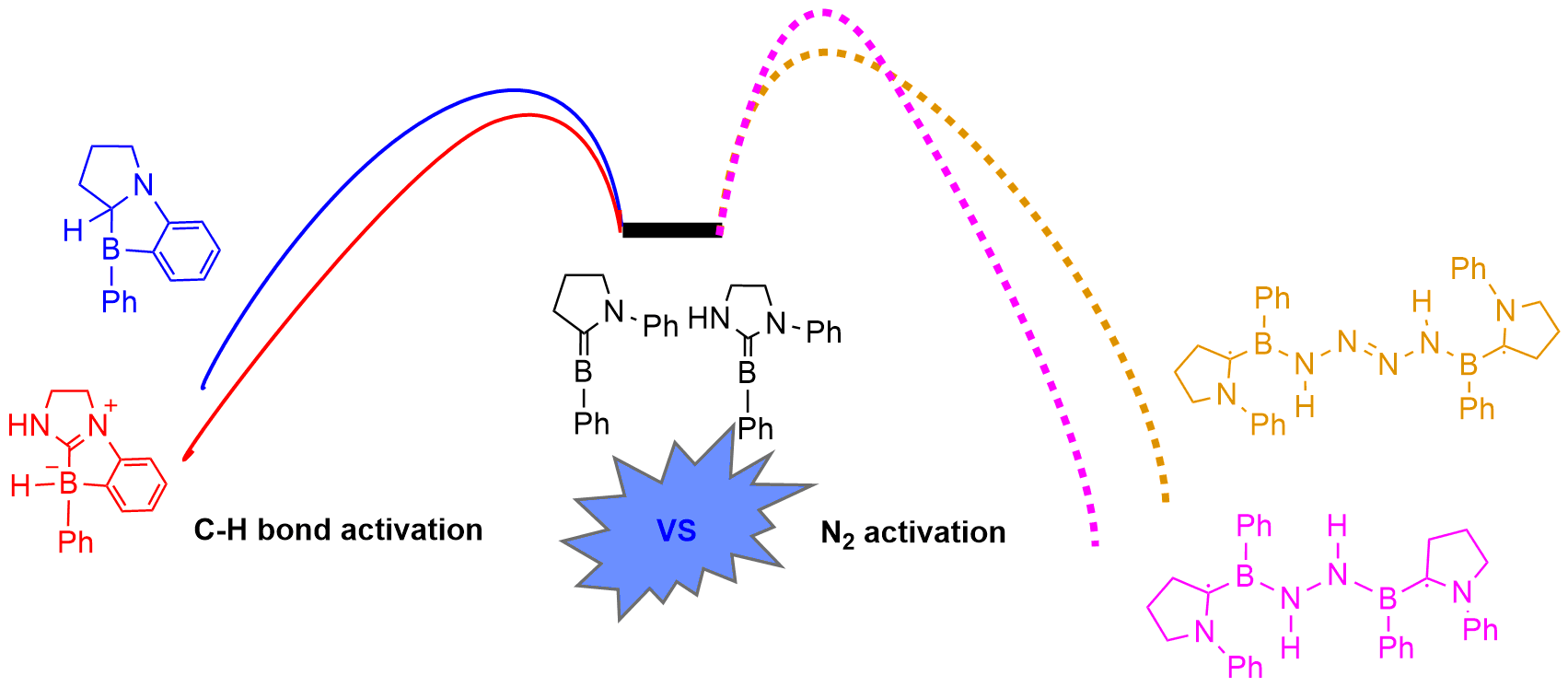
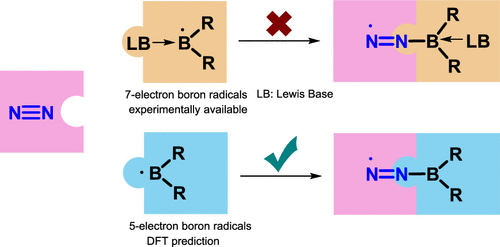
Activation of dinitrogen (N2) under mild conditions has been a particularly challenging project for decades, owing to the highly strong N≡N triple bond. In recent years, the main group species have emerged as a prominent strategy in the field of dinitrogen activation, but the reported examples remain particularly rare compared with transition metal complexes. Herein, we performed a comprehensive density functional theory (DFT) calculation of N2 activation by boron radical cations.