Predicting Small Molecule Activations Including Dinitrogen Based on an Inorganic Benzene B4N2 Framework
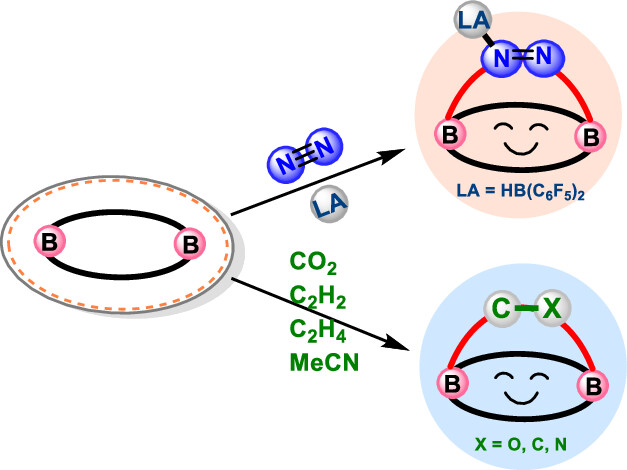
Although main group species have emerged in the field of dinitrogen activation in recent years, the reported examples are particularly rare in comparison with transition metal complexes due to their significant challenges. Herein, we demonstrate a [4 + 2] cycloaddition reaction of N2 (with an activation energy as low as 12.5 kcal mol–1) initiated by an inorganic benzene via density functional theory calculations. Such N2 activation is supported by the elongated nitrogen–nitrogen bond distance (dNN), decreased vibration frequency (νNN), and weakened Wiberg bond index (WBINN). Subsequently, the “push-pull” electronic effect, formed by introducing a Lewis acid, HB(C6F5)2, facilitates the generation of thermodynamically more stable products. In addition, this inorganic benzene could also be used to activate a series of small molecules, including carbon dioxide, acetylene, ethylene, and acetonitrile with reaction barriers ranging from 4.7 to 11.6 kcal mol–1. Our findings provide an alternative approach to N2 activation and functionalization, theoretically validating the feasibility of the dual Lewis acid strategy for dinitrogen activation.
