Predicting an Antiaromatic Benzene Ring in the Ground State Caused by Hyperconjugation
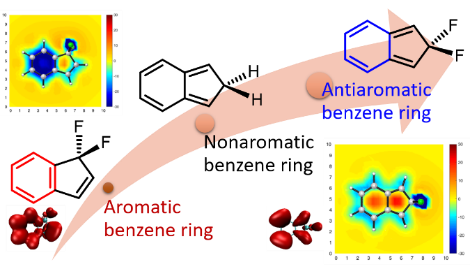
Benzene, the prototype of aromatics, has six equivalent C‐C bonds (1.397 Å), which are intermediate between a C‐C double bond and a C‐C single bond. For over 80 years, chemists have spent much effort on freezing a localized structure to obtain a distorted bond‐length alternating benzene ring in the ground state, leading to various localized trisannelated benzene rings. However, most of the central benzene rings are still aromatic or nonaromatic. Here we report an antiaromatic benzene ring caused by hyperconjugation. Specifically, symmetric annulation of 5,5‐difluorocyclopentadiene results in an antiaromatic benzene ring, which is supported by various aromaticity indices, including nucleus independent chemical shift, anisotropy of the induced current density, π‐separated electron‐localization function and heat of hydrogenation. Our findings highlight a strong power of hyperconjugation, a “weak” interaction in organic chemistry, paving the way for designing and realizing more novel (anti)aromatics.
