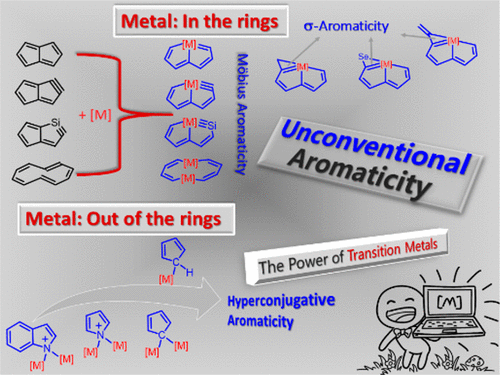
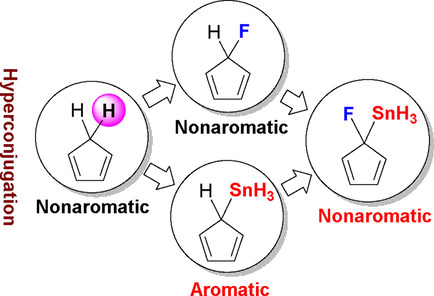
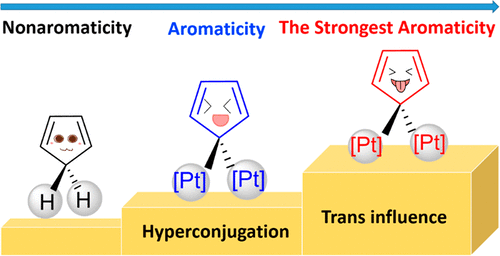
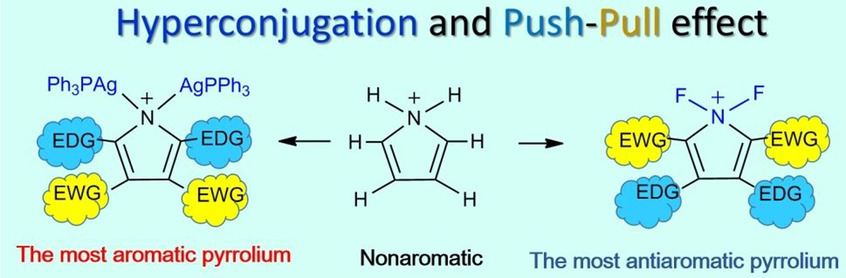
Predicting an Antiaromatic Benzene Ring in the Ground State Caused by Hyperconjugation
Submitted by Jun Zhu on Tue, 10/29/2019 - 22:58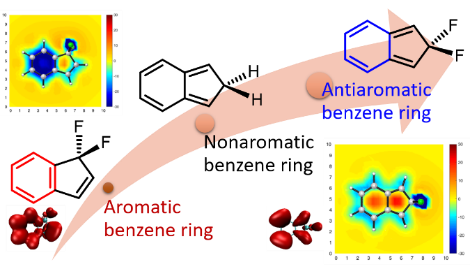
Benzene, the prototype of aromatics, has six equivalent C‐C bonds (1.397 Å), which are intermediate between a C‐C double bond and a C‐C single bond. For over 80 years, chemists have spent much effort on freezing a localized structure to obtain a distorted bond‐length alternating benzene ring in the ground state, leading to various localized trisannelated benzene rings. However, most of the central benzene rings are still aromatic or nonaromatic. Here we report an antiaromatic benzene ring caused by hyperconjugation.