Solvent-driven Interconversion of Pyridine Dicarbanion-bonded Ag13 Nanocluster Isomers
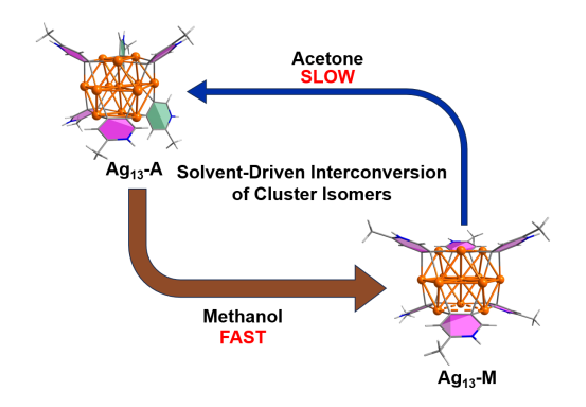
Solvent screening is pivotal in the optimization of reaction conditions for metal-catalyzed reactions. However, to date little is known about the role of solvent effects in driving in situ structural evolution of metal-containing species towards polynuclear organometallic cluster intermediates and determining their reactivity. We herein isolate two distinct thirteen-membered silver cluster isomers, Ag13-A and Ag13-M, in acetone and methanol, respectively. Significantly, we demonstrate the interconversion of these isomers facilitated by solvent manipulation, revealing a novel aspect of organometallic nanoclusters. Mechanistic studies on the solvent-driven Ag13-A to Ag13-M transformation reveal a low activation energy of 22.39 ± 0.81 kcal mol-1 and a positive activation entropy, which together with theoretical investigations substantiate that the isomeric transformation involves counter anion dissociation, subsequent twisting of peripheral pyridine dicarbanion ligands, and eventual re-coordination of the counter anions. The observed differences in thermal stability and reactivity between two isomers are attributed to variations in Ag-Ag interactions and surface ligand arrangements, underscoring the critical role of solvent selection in affecting the whole organic transformation through in situ formed clusters. These findings demonstrate a new perspective on solvent dependency in metal-catalyzed organic transformations, foreseeing the necessity of considering organometallic cluster intermediates in solvent screening.
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202506860
