Crystalline Arylstibinidene Chalcogenides: Heavier Congeners of Aromatic Nitroso Compounds
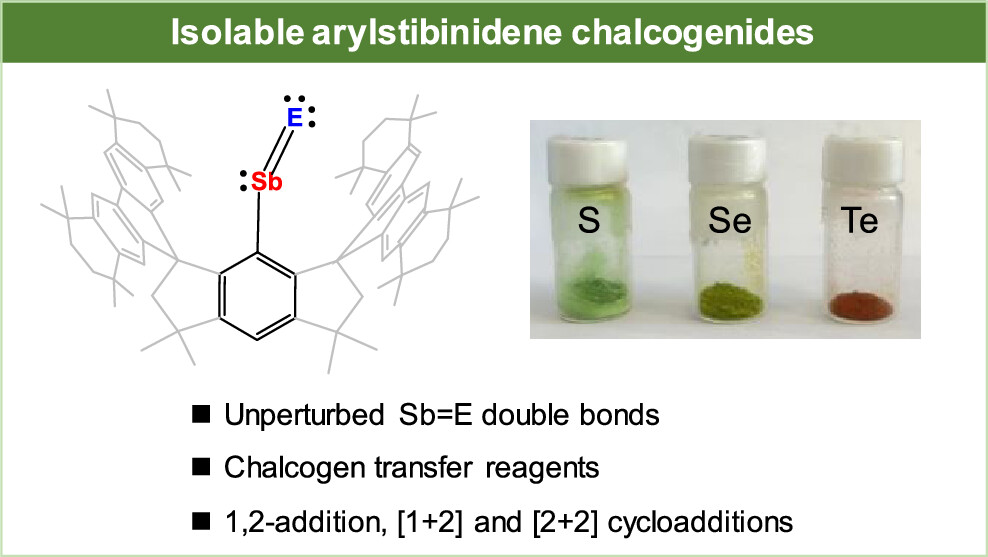
Nitroso compounds, R-N═O, containing N═O double bonds are ubiquitous and widely utilized in organic synthesis. In contrast, heavier congeners of nitroso compounds, namely pnictinidene chalcogenides R-Pn = E (Pn = P, As, Sb, Bi; E = O, S, Se, Te), are highly reactive and scarce. They have been stabilized in the coordination sphere of Lewis acid/base or by pronounced contribution from resonance structures, whereas free species with unperturbed pnictogen-chalcogen double bonds remains elusive. In this work, we report the isolation and characterization of arylstibinidene chalcogenides, which are the first heavier congeners of aromatic nitroso compounds. They are facilely synthesized through the salt metathesis reactions of aryldichlorostibane and dilithium chalcogenides. They bear unperturbed Sb═E (E = S, Se and Te) double bonds due to poor orbital overlap between the C 2p orbitals of the phenyl ring of the substituent and the Sb 5p orbitals. Moreover, they show versatile reactivity, including acting as chalcogen atom transfer reagents and reacting with small molecules via (cyclo)addition.
