Carbon-halogen bond activation by a structurally constrained phosphorus(III) platform
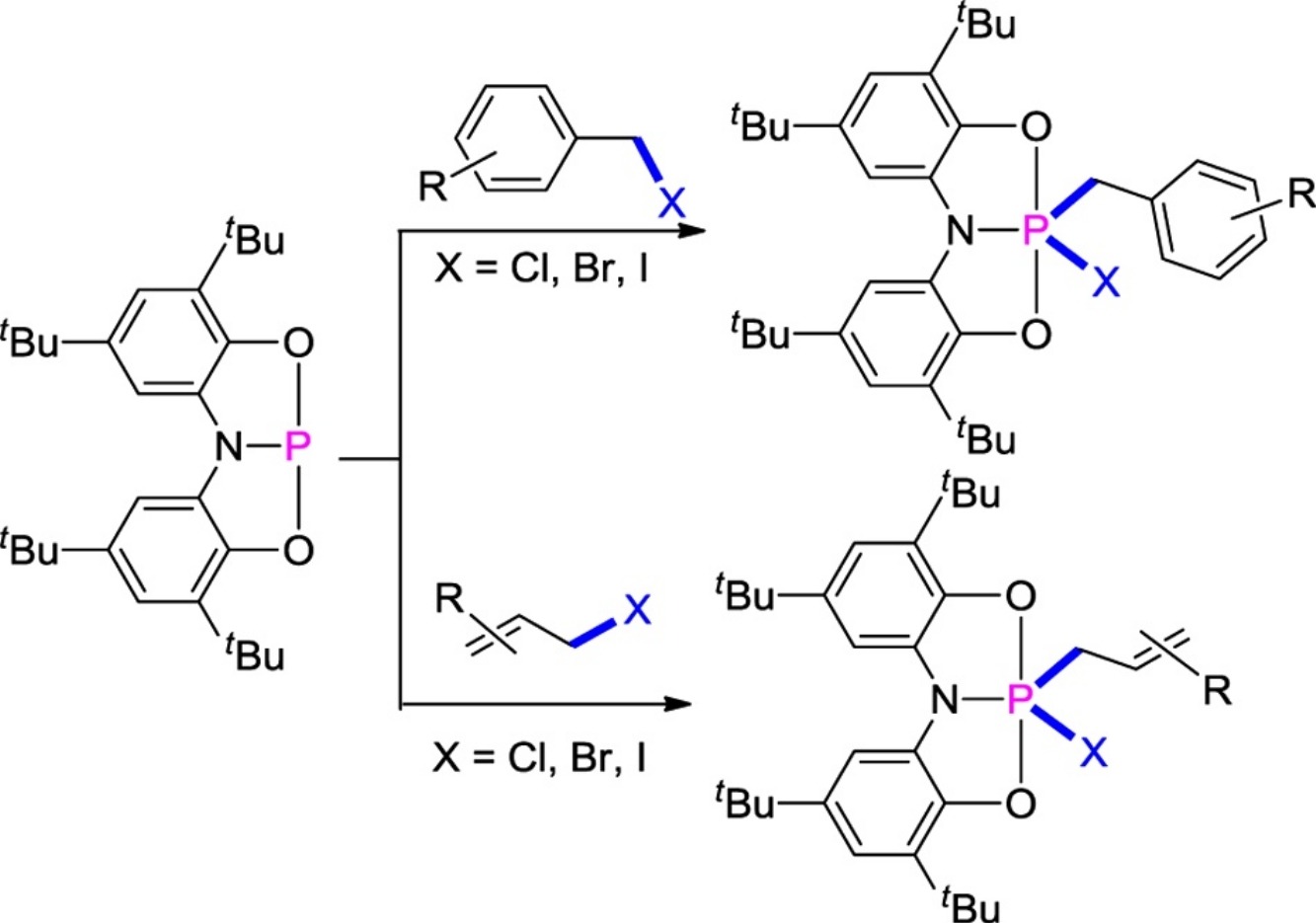
The σ-bond activation by main group element has received enormous attention from theoretical and experimental chemists. Here, the reaction of C-X (X = Cl, Br, I) bonds in benzyl and allyl halides with a pincer-type phosphorus(III) species was reported. A series of structurally robust phosphorus(V) compounds were formed via the formal oxidative addition reactions of C-X bonds to the phosphorus(III) center. Density functional theory calculations show that the nucleophilic addition process is more favorable than the direct oxidative addition mechanism. Isomerization of bent structures of phosphorus(III) compound to poorly nucleophilic compounds to undergo further C-X bond activation can be rationalized by frontier molecule orbital analysis. This study not only provides a deep understanding of the reactivity of phosphorus(III) species but also demonstrates a potential of main group elements for the small-molecule activation.
https://www.sciencedirect.com/science/article/pii/S1001841720306380
