A novel metal-wingtip pentalene system: the synthesis, structure, and reactivity of non-aromatic wingtip osmapentalenes
Submitted by Jun Zhu on Wed, 04/23/2025 - 14:59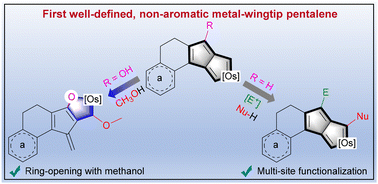
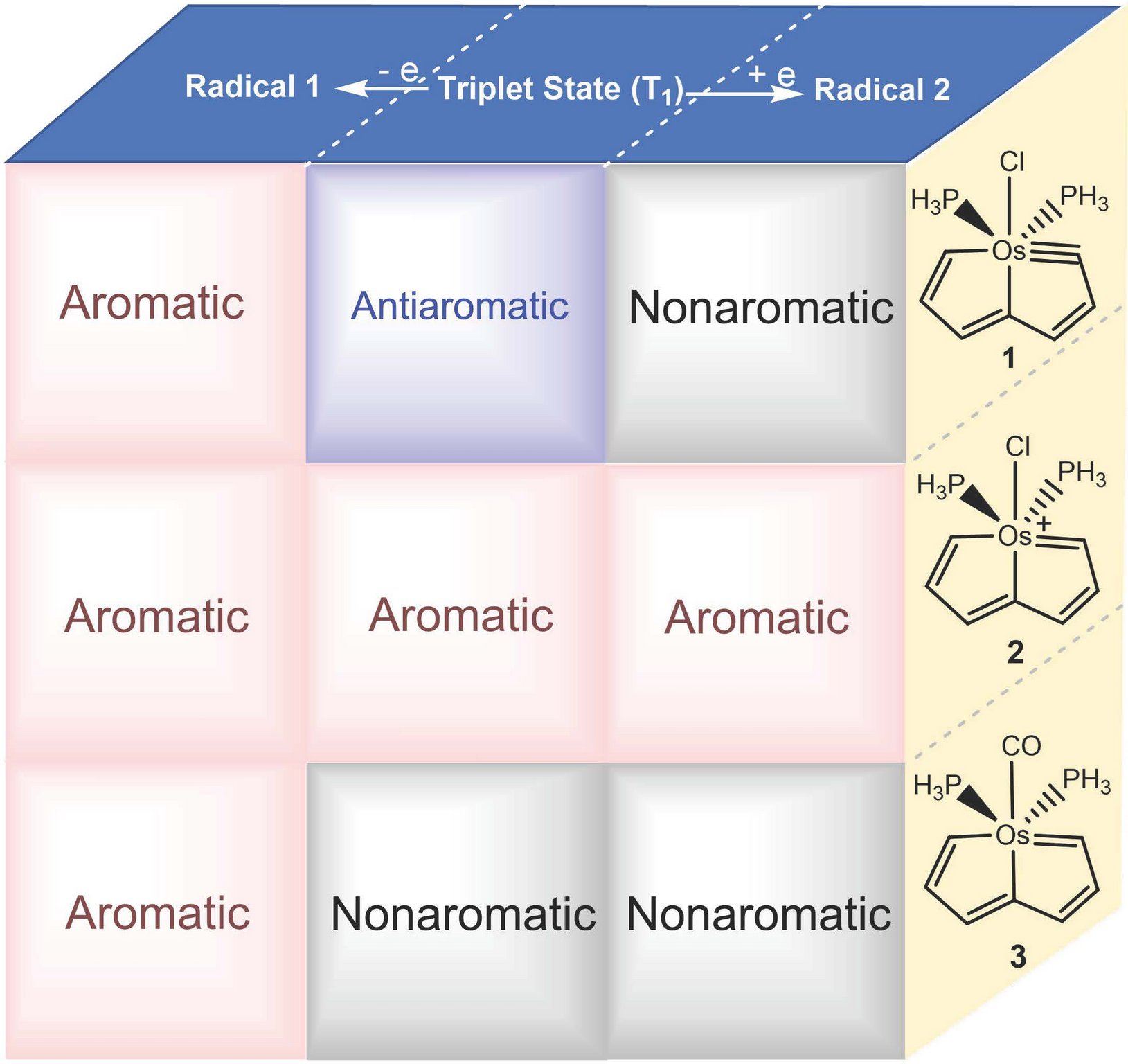
Since the concept of aromaticity was first introduced in transition metal complexes, metals have become a crucial component for modulating aromaticity, leading to a variety of structural frameworks. Initial studies successfully achieved aromatic pentalene dianions through metal-ion coordination, and recent advancements in bridgehead metallapentalenes have demonstrated the transformation of antiaromaticity into aromaticity.