Dialumene as a Dimeric or Monomeric Al Synthon for C–F Activation in Monofluorobenzene
Submitted by Jun Zhu on Fri, 08/30/2024 - 20:34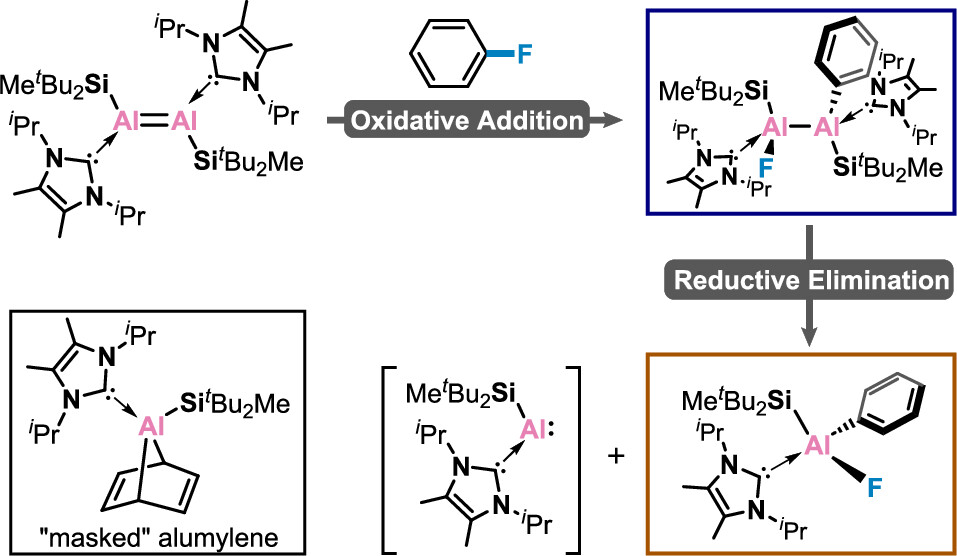
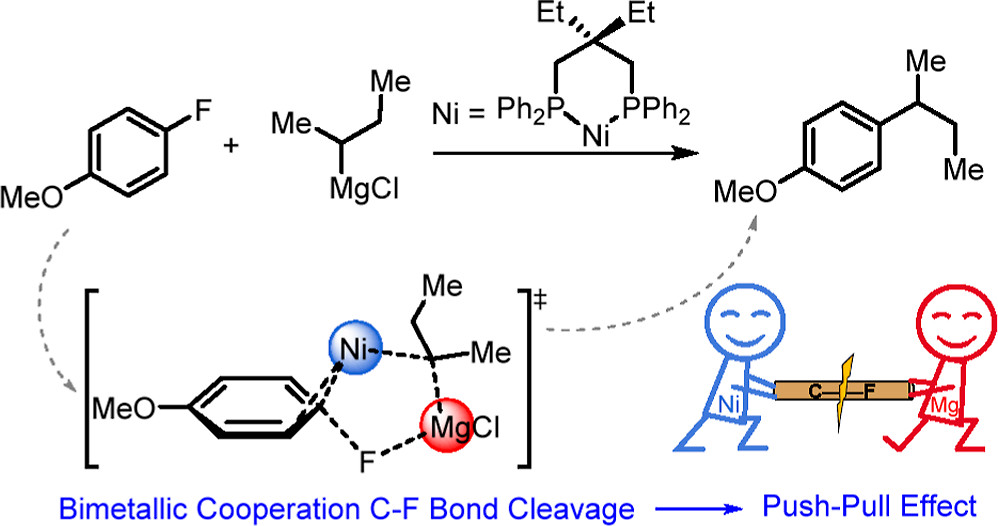
The activation of C–F bonds has long been regarded as the subject of research in organometallic chemistry, given their synthetic relevance and the fact that fluorine is the most abundant halogen in the Earth’s crust. However, C–F bond activation remains a largely unsolved challenge due to the high bond dissociation energies, which was historically dominated by transition metal complexes. Main group elements that can cleave unactivated monofluorobenzene are still quite rare and restricted to s-block complexes with a biphilic nature.