Main group metal–ligand cooperation of N-heterocyclic germylene: an efficient catalyst for hydroboration of carbonyl compounds
Submitted by Jun Zhu on Wed, 12/21/2016 - 13:51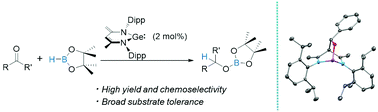
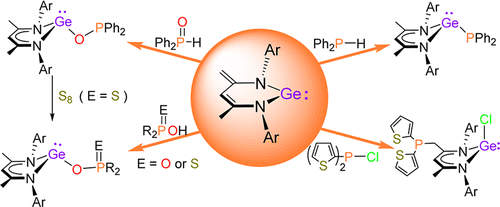
N-heterocyclic ylide-like germylene effectively promotes the hydroboration of aldehydes and ketones under mild conditions with broad substrate tolerance, operational simplicity of procedure and excellent yields. A key intermediate in this catalytic system featuring a bicyclo[2,2,2]octane-like core has been successfully isolated and characterized, suggesting a new type of mechanism that involves the activation mode that mimics that of transition metal catalysts.