Pd-catalyzed highly regio-, diastereo-, and enantioselective allylic alkylation of α-fluorophosphonates
Submitted by Jun Zhu on Tue, 06/03/2014 - 13:07
Authors:
Ying Huang, Qing-Song Zhang, Ping Fang,* Tie-Gen Chen, Jun Zhu, Xue-Long Hou*
Journal:
Chem. Commun.
Year:
2014
Volume:
50
FirstPage-LastPage:
6751-6753
TOC:

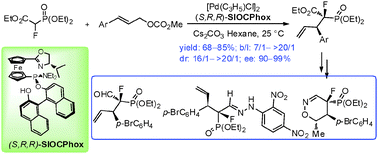
Abstract:
Highly efficient Pd-catalyzed asymmetric allylic alkylation reaction of ethyl-2-fluoro-2-(diethoxyphosphoryl)acetate with monosubstituted allylic substrates has been developed, affording corresponding α-fluorophosphonates with two chiral centers in high regio-, diastereo- and enantio-selectivities. The usefulness of the products in organic synthesis has been demonstrated.
http://pubs.rsc.org/en/content/articlelanding/2014/cc/c4cc02158d#!divAbstract
Doi:
10.1039/C4CC02158D
