NO-induced adaptive aromaticity in furan, thiophene and selenophene
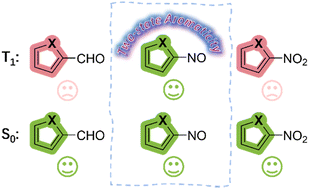
The concept of adaptive aromaticity, which denotes two-state aromaticity in both the lowest singlet and triplet states, stands in marked contrast to the traditional one-state aromaticity governed by the Hückel and Baird rules. Nonetheless, organic compounds exhibiting adaptive aromaticity remain particularly rare. In this study, we demonstrate adaptive aromaticity in a range of organic compounds, namely, furan, thiophene and selenophene, via the introduction of a substituent –NO group, which is supported by various aromaticity indices including isomerization stabilization energy (ISE), nucleus-independent chemical shift (NICS), anisotropy of the induced current density (AICD) and electron density of the delocalized bond (EDDB). Further study suggests that the aromaticity in the lowest triplet state of these species is caused by electronic excitation from in-plane π to out-of-plane π* molecular orbitals in the substituents. All these findings significantly enrich the family of adaptive aromaticity, contributing to the development of aromatic chemistry.
https://pubs.rsc.org/en/content/articlelanding/2025/nj/d5nj00492f
