Probing σ-Aromaticity-Driven Ring Contraction of Metallabenzocyclobutadiene to Metallabenzocyclopropene
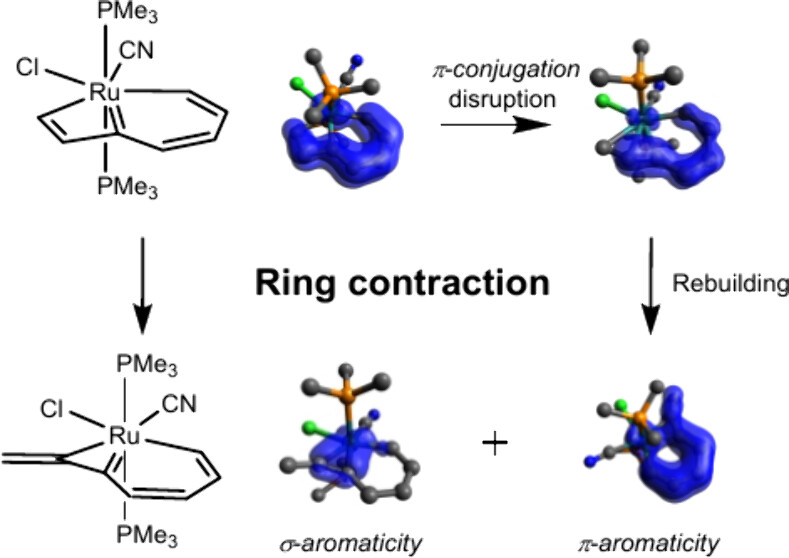
Ring contraction of metallacyclobutadiene to metallacyclopropene is rare because of the increasing strain from a four-membered ring to a three-membered one. Here we demonstrate a new series of reactions of metallabenzocyclobutadiene to metallabenzocyclopropene via density functional theory calculations. The results suggest that these reactions are thermodynamically favorable ranging from −17.4 to −29.4 kcal mol–1, and a low reaction barrier (10.3 kcal mol–1) is achieved when the metal center is Ru and the ligands are one cyanide and one chloride. Further analysis suggests that a strengthened binding energy helps stabilize the transition state in the protonation process. The aromaticity during the reaction was investigated using the electron density of delocalized bonds (EDDB), isomerization stabilization energy, and isodesmic reactions. The EDDB shows that the π-conjugation is disrupted in the intermediate, and then σ-aromaticity is generated and dominant in the products. Our findings could be helpful for experimentalists in developing novel ring contraction reactions driven by aromaticity.
