π-Aromaticity Dominating in a Saturated Ring: Neutral Aromatic Silicon Analogues of Cyclobutane-1,3-diyls
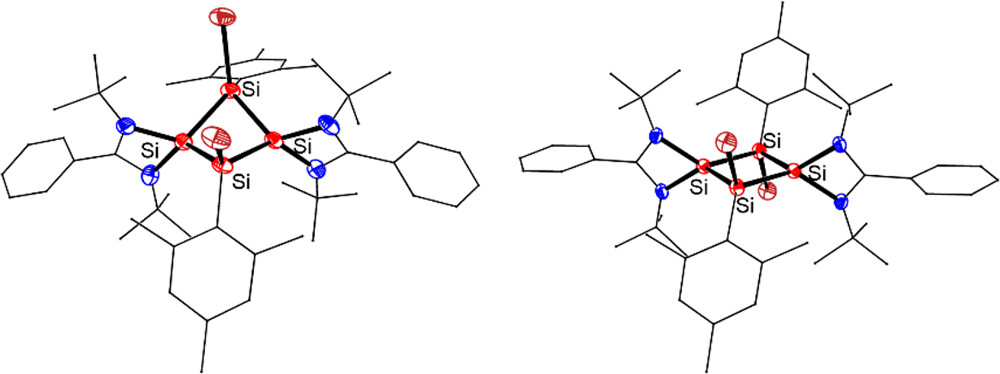
The synthesis, structures, and reactivity of the first neutral 2π-aromatic Si4 rings [LSiSiAr(X)]2 (3: X = Br; 4: X = Cl; L = PhC(NtBu)2, Ar = 2,4,6-Me3C6H2) were described. Compounds 3 and 4 were obtained by 1,3-halogenation of tetrasilacyclobutadiene (LSiSiAr)2 (2), which was prepared by the reductive cross-coupling of trisilane (ArSiCl2)2SiHAr with two equiv of chlorosilylene LSiCl. The reaction of 3 with two equiv of PhLi yielded the corresponding substitution Si4 ring [LSiSiAr(Ph)]2 (5). Single-crystal X-ray diffraction analysis of 3 disclosed that it adopts both puckered (3a) and planar (3b) structures in the solid state, whereas 4 and 5 exhibit only a puckered structure. DFT calculations suggested that the puckered 3a features almost the same electronic structure with fully delocalized 2π planar 3b. The dominant 2π-aromaticity of 3 in a σ-frame has been demonstrated by DFT calculations, providing the first example of aromatics featuring both planar and puckered structures.
