Isolation of a carbon nanohoop with Möbius topology
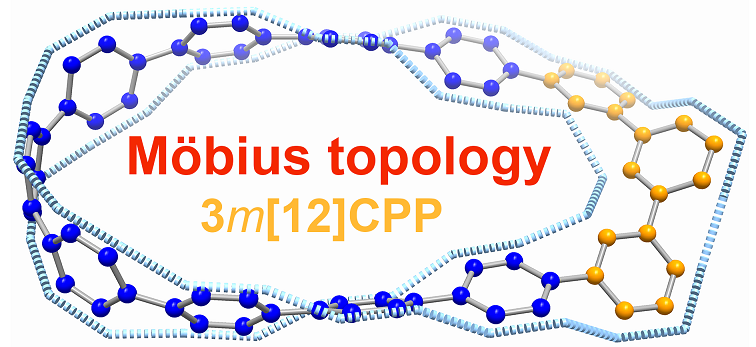
Carbon nanohoop, a class of constrained molecular architecture consisting of linked arene units, has attracted considerable interest from both experimental and theoretical chemists due to their synthetic challenge and aesthetic architectures. Another fascinating and synthetically challenging species, the Möbius-type molecule, has been attracting the scientific community with its elegant structure and aromaticity. Thus, combining two things together, synthesizing a carbon nanohoop with Möbius topology remains more challenging to date. Here we report a cyclophenylene featuring Möbius strip characterized by X-ray crystallography. Theoretical calculations reveal that such type of nanohoop is fully conjugated systems with electrons delocalized both in π sextets and the bridging carbon-carbon bonds. This work highlights that the manipulation of phenylene connection in a carbon nanohoop can help obtain more delicate and aesthetic molecular architectures.
