Reaction mechanisms of iron(iii) catalyzed carbonyl–olefin metatheses in 2,5- and 3,5-hexadienals: significant substituent and aromaticity effects
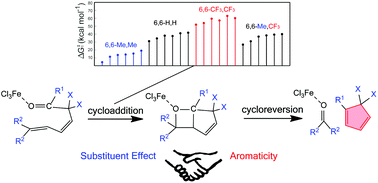
Olefin metathesis is a fundamental organic reaction of great importance that led to the 2005 Nobel Prize in Chemistry. As a variation of olefin–olefin metathesis, carbonyl–olefin metathesis (COM) is less developed, but still significant progress has been made recently. However, how the aromaticity affects the reaction mechanisms remains unclear. Here we perform density functional theory calculations on iron(III) catalyzed COM in 2,5- and 3,5-hexadienals. Natural population analyses in conjunction with molecular geometries reveal two different activation patterns for the cycloaddition process which depend on the substituent. Moreover, with appropriate substituents the COM reactions could be both thermodynamically and kinetically feasible. By comparison with 33 models, significant effects of both substituent and aromaticity on the reaction mechanisms are observed in the cycloaddition and cycloreversion processes, respectively, which could be useful for experimental chemists to efficiently test more COM reactions.
