β-Hydrogen Elimination of Five-Membered-Ring Metallacycles. Is It Possible?
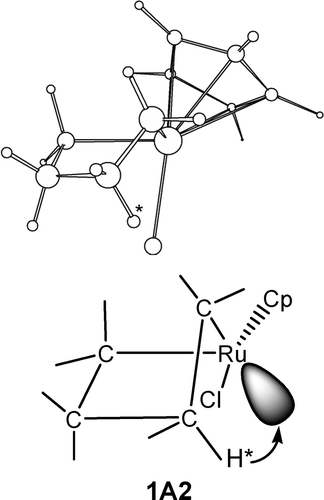
B3LYP density functional theory calculations have been carried out to examine the structural and energetic aspects of β-hydrogen elimination in several metallacyclic complexes of ruthenium and platinum. Factors affecting barriers of the elimination reactions have been examined. It was found that favorable structural arrangements, in which the transferring β-hydrogen is in close proximity to the metal center, for β-hydrogen elimination exist in certain ring conformations of metallacyclic complexes. However, favorable electronic requirements, which allow the transferring β-hydrogen to have effective orbital overlap with the hydride-receiving unoccupied orbital from the metal center, cannot always be achieved. Calculations show that β-hydrogen elimination of several five- and six-membered-ring, 16-electron ruthenium complexes occurs easily. The corresponding reactions of platinum complexes were found to be difficult.
