Theoretical study on reactions of O3+ and N2: novel routes to dinitrogen bond activation
Submitted by Jun Zhu on Thu, 10/31/2013 - 18:04
Authors:
Jun Zhu, Zexing Cao,* Qianer Zhang
Journal:
Chem. Phys. Lett.
Year:
2003
Volume:
377
FirstPage-LastPage:
184–188
TOC:

Abstract:
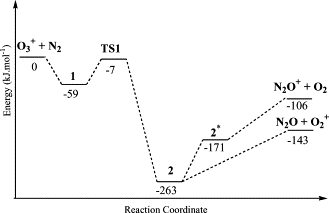
Plausible mechanisms for reactions of the ionized O-3 with N-2 are studied by DFT and electron correlation methods. Calculations show that formation of the primary products O-2(+) + N2O and N2O+ + O-2 arises from an intermediate [O-2...ON2](+) in the ground state and its charge-transfer excited state, respectively. New routes to NO2 + NO+ through an intermediate [ON2...O-2](+) and to [ON...NO](+) + O-2 via the reactions of O-3(+) with N2O are proposed. The former has a high barrier, while the latter is facile process with a small barrier of 20 kJ mol(-1) and a huge exothermicity of 502 kJ mol-1.
Doi:
10.1016/S0009-2614(03)01131-X
