Theoretical studies on structures and spectroscopic properties of nitryl halogenides
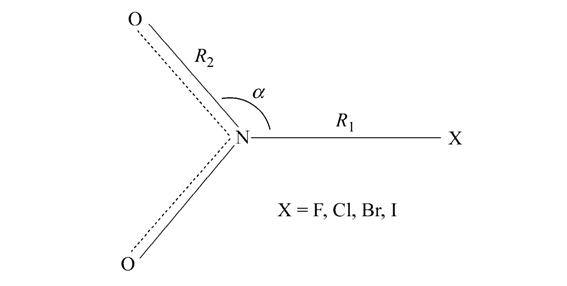
Density functional theory with the B3LYP functional is used to calculate the equilibrium geometries and harmonic vibrational frequencies of nitryl halogenides XNO2 and XONO (X = F, Cl, Br, I). Stabilities and isomerizations of these isomers are investigated. Dissociation energies of the X-N bond in XNO2 are predicted at the B3LYP/6-311G* and QCISD(T)/ce-pvTZ levels. The electronic transition energies of the most stable XNO2 species have been estimated by time-dependent B3LYP calculations. The electron promotion of a nonbonding electron of the halogen atom X in XNO2 into a pi* orbital on the NO2 moiety, i.e., the n-->sigma* electron excitation, is responsible for the photodissociation of the X-N bond.
